Radiation-induced Syringomatous Carcinoma: A Case Report
Images
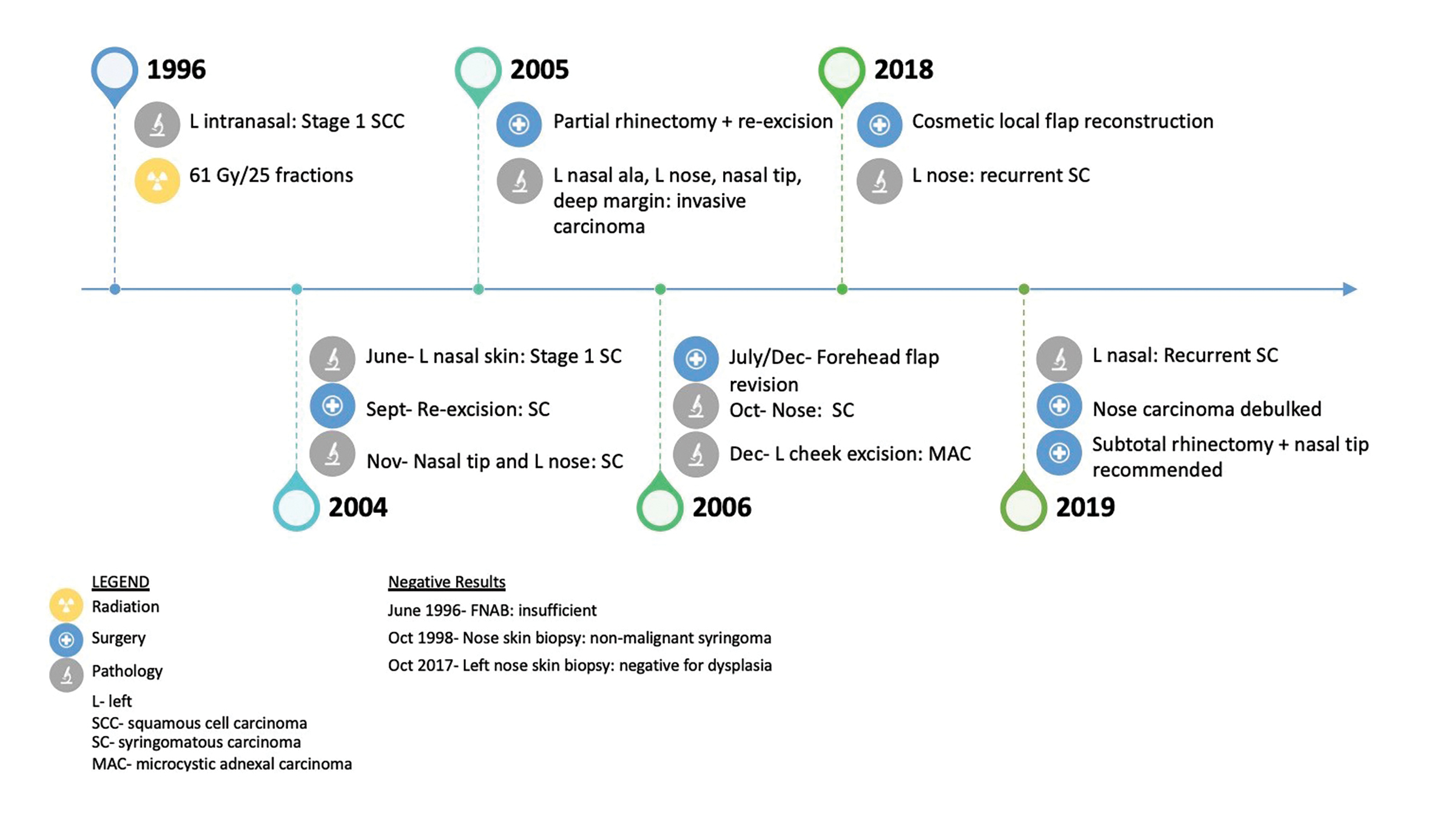
CASE SUMMARY
A 51-year-old patient presented to her family physician with a 14-year history of a tender, “pimple-like” lesion on her left nostril (see Figure 1 for timeline). Biopsy of the area confirmed squamous cell carcinoma (SCC), stage 1.1 Computed tomography (CT) of the face and neck reported a small soft-tissue mass along the left nose extending to (but not crossing) midline and no nodal involvement. Treatment involved 61 Gy in 25 daily fractions using the parallel-opposed pair (POP) technique with 4 MV. The radiation field covered the left nose with superior margin falling off the nose, onto the upper lip, and including the left nasolabial fold and septum.
Eight years later, the patient presented again with a lesion in the left nostril. Pathology and external consultation confirmed syringomatous carcinoma due to the infiltrative growth pattern of a dermal tumor. Treatment included re-excision of positive margins, partial rhinectomy, and forehead flap for nasal reconstruction. Fourteen years after the diagnosis of syringomatous carcinoma, the patient presented for surgery to improve cosmesis and relieve breathing obstruction. Biopsy of the left nose revealed recurrent syringomatous carcinoma that extended deeply up to but not invading the level of the cartilage. The patient was not considered a good candidate for further radiation therapy primarily due to previous radiation and the relatively indolent nature of her disease. A subtotal rhinectomy was therefore recommended.
DIAGNOSIS
Radiation-induced syringomatous carcinoma of the left nose. Differential diagnosis included fibroblastic/desmoplastic trichoepithelioma, basal cell carcinoma, syringoma, and syringomatous carcinoma.
DISCUSSION
Syringomatous carcinoma (SC) is a rare, slow-growing, heterogenous tumor of sweat gland origin.2 Rarely metastatic, the most common sites include the head and neck regions, particularly the scalp.3 Histologically, the absence of keratinizing cysts and squamous differentiation (ie, islands with parakeratotic keratinization) distinguish SC from other sclerosing adnexal tumors such as microcystic adnexal carcinoma (MAC) and squamoid eccrine ductal carcinoma (SEDC), respectively.3 Historically, the literature did not consistently distinguish between SC and MAC. Providing an accurate estimate of incidence for SC is therefore challenging. More generally, skin adnexal carcinomas have an incidence rate of 5.1 per 1 million person-years.4
Due to the rare nature of SC, its pathogenesis is not yet well understood. Development of a closely related neoplasm, MAC, however, has been linked to patients who previously received radiation treatment.5-7 Exposure to radiation, whether therapeutic or otherwise, causes changes in the DNA of normal tissues, which can lead to tumorigenesis.8 The site of previous radiation is at highest risk of radiation-induced secondary malignancy due to the high therapeutic radiation doses administered.9 Secondary radiation-induced malignancies can occur decades after the initial radiation treatment.9 The documented link between previous radiation and developing MAC suggests that mutagenesis caused by radiation treatment for this patient’s squamous cell carcinoma likely contributed to the development of syringomatous carcinoma.
In the reported case, the patient had no other comorbidities. Her risk factors included a 50-pack-year smoking history (she quit in 1972), and minimal alcohol consumption. Her family history was significant for a brother who died from an unknown type of cancer at the age of 65, and a sister who died from a brain tumor at the age of 42.
In addition to the patient’s ineligibility for further radiation, syringomatous carcinomas are thought to be resistant to radiation due to their slow-growing nature. Wide surgical excision was performed in this case, as it is the first-line treatment. It has been reported in the literature, however, that Mohs micrographic surgery allows for a lower rate of recurrence.10 Micrographic surgery involves removing layers of tissue in stages and examining them microscopically for cancerous cells. The process is repeated until no cancerous cells remain, resulting in up to 99% cure rates for skin malignancies that have not been previously treated.11 Mohs micrographic surgery is also considered the first-line treatment for skin malignancies previously treated with radiation, and should be considered in future cases.11
CONCLUSION
Although radiation-induced MAC has been reported, there are no previous reports of radiation-induced syringomatous carcinoma. This report presents a unique case of likely radiation-induced syringomatous carcinoma in a patient previously treated with radiation for a left intranasal squamous cell carcinoma. It highlights the difficult balance between radiation therapy as an effective oncologic treatment and as a risk factor for the development of secondary malignancies. It is additionally challenging to treat radiation-induced malignancies, as radiation therapy is generally no longer an effective treatment option. In delicate locations such as the head and neck, advanced surgical techniques such as Mohs micrographic surgery may be required to treat cutaneous radiation-induced malignancies.
REFERENCES
- Amin MB, Edge S, Greene F, et al. AJCC Cancer Staging Manual 8th ed. Springer Int Publ. 2017;8. doi:10.1007/978-3-319-40618-3
- Freeman RG, Winkelmann RK. Basal cell tumor with eccrine differentiation (eccrine epithelioma). JAMA Dermatol. 1969;100(2):234-242. doi:10.1001/archderm.1969.01610260110021
- Frouin E, Vignon-Pennamen MD, Balme B, et al. Anatomoclinical study of 30 cases of sclerosing sweat duct carcinomas (microcystic adnexal carcinoma, syringomatous carcinoma and squamoid eccrine ductal carcinoma). J Eur Acad Dermatol Venereol. 2015;29(10):1978-1994. doi:10.1111/jdv.13127
- Blake PW, Bradford PT, Devesa SS, Toro JR. Cutaneous appendageal carcinoma incidence and survival patterns in the United States: A population-based study. Arch Dermatol. 2010;146(6):625-632. doi:10.1001/archdermatol.2010.105
- Antley CA, Carney M, Smoller BR. Microcystic adnexal carcinoma arising in the setting of previous radiation therapy. J Cutan Pathol. 1999;26(1):48-50. doi:10.1111/j.1600-0560.1999.tb01790.x
- Abbate M, Zeitouni NC, Seyler M, Hicks W, Loree T, Cheney RT. Clinical course, risk factors, and treatment of microcystic adnexal carcinoma: a short series report. Dermatol Surg. 2003;29(10):1035-1038.
- Snow S, Madjar DD, Hardy S, et al. Microcystic adnexal carcinoma: report of 13 cases and review of the literature. Dermatol Surg. 2001;27(4):401-408.
- Braunstein S, Nakamura JL. Radiotherapy-induced malignancies: review of clinical features, pathobiology, and evolving approaches for mitigating risk. Front Oncol. 2013;3:73. doi:10.3389/fonc.2013.00073
- Morton LM, Onel K, Curtis RE, Hungate EA, Armstrong GT. The rising incidence of second cancers: patterns of occurrence and identification of risk factors for children and adults. Am Soc Clin Oncol Educ book Am Soc Clin Oncol Annu Meet. 2014:e57-67. doi:10.14694/EdBook_AM.2014.34.e57
- Chiller K, Passaro D, Scheuller M, Singer M, McCalmont T, Grekin RC. Microcystic adnexal carcinoma: forty-eight cases, their treatment, and their outcome. Arch Dermatol. 2000;136(11):1355-1359. doi:10.1001/archderm.136.11.1355
- Kauvar A. Mohs: The Gold Standard. The Skin Cancer Foundation. Accessed September 18, 2019. https://www.skincancer.org/treatment-resources/mohs-surgery/mohs-the-gold-standard.
Citation
O’Neill S, Zayed S, Ahmad B. Radiation-induced Syringomatous Carcinoma: A Case Report. Appl Radiat Oncol. 2020;(3):36-37.
September 9, 2020
