Teaching Case: Radiation Myonecrosis Following Stereotactic Body Radiation Therapy in Metastatic Renal Cell Carcinoma
Images
Abstract
Cases of radiation-induced myonecrosis are exceedingly uncommon in radiation literature. We present a rare case of radiation myonecrosis in a 39-year-old woman with metastatic renal cell carcinoma (RCC) on concurrent immunotherapy, following 40 Gy in 5 fractions of radiation to a paravertebral muscle metastasis in the neck. We describe a 1-year timeline wherein the patient receives radiation therapy, develops clinical signs of radiation myonecrosis, and then shows radiographic resolution of the lesion followed by radiographic evidence of radiation myonecrosis at the treatment site. The patient improved clinically within 1 month following radiographic diagnosis. We add this to the collection of published radiation-induced myonecrosis cases to caution radiation experts about potential side effects of radiation therapy.
Keywords: radiation myonecrosis, toxicity, renal cell carcinoma, RCC, immunotherapy, stereotactic body radiation therapy, SBRT
Introduction
Renal cell carcinoma (RCC) is the 7th most diagnosed cancer in the developed world and one of the fastest-growing cancer diagnoses in the US.1 It comprises many histopathologic variants, the most common being the clear cell variant, with the rhabdoid variant associated with higher mortality rate and poorer prognosis.2 Although no clear explanation exists, laboratory experiments have shown that RCC is also relatively radioresistant and evidence suggests that hypofractionated radiation therapy with higher doses and fewer fractions are necessary to overcome these tumor cells.3,4 Thus, stereotactic body radiation therapy (SBRT) is now preferred over conventional radiation therapy.4,5 We present a rare case of radiation myonecrosis in a patient with metastatic RCC, treated with concurrent immunotherapy and SBRT. This is the first case of radiation myonecrosis following radiation therapy for RCC, and only the second case of radiation myonecrosis following SBRT.
Case Summary
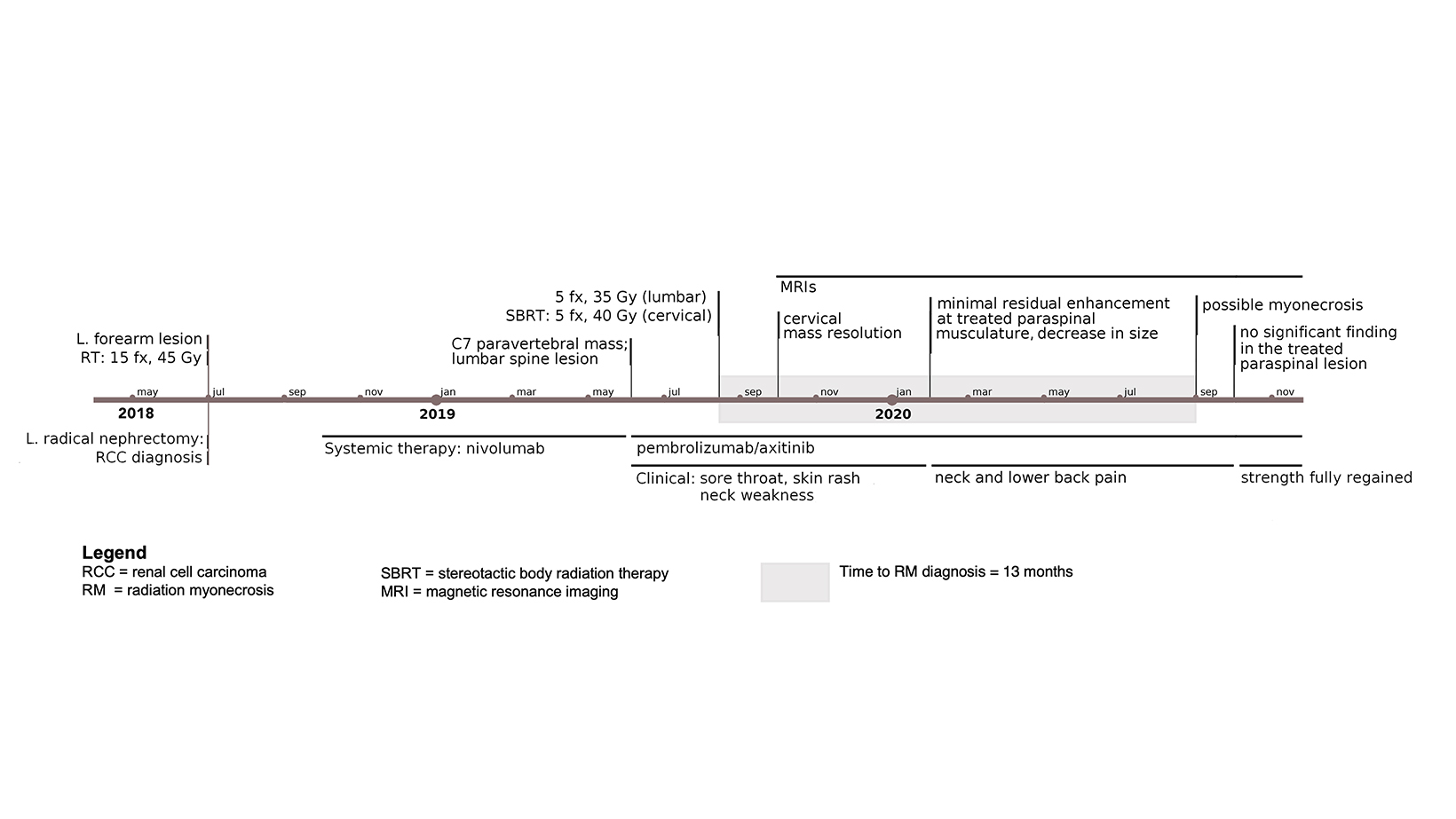
Our patient is a 39-year-old woman diagnosed with metastatic RCC after resection of a left-forearm mass in July 2018. Initial x-rays followed by MRI revealed a soft-tissue mass and ulnar lytic lesion concerning for malignancy, and positron emission tomography / computed tomography (PET/CT) revealed a left renal mass presumed to be her primary site of disease. Her disease course/timeline is depicted in Figure 1. Ultrasound-guided biopsy followed by left radical nephrectomy confirmed clear cell RCC. Her forearm received postoperative RT to 45 Gy in 15 fractions.
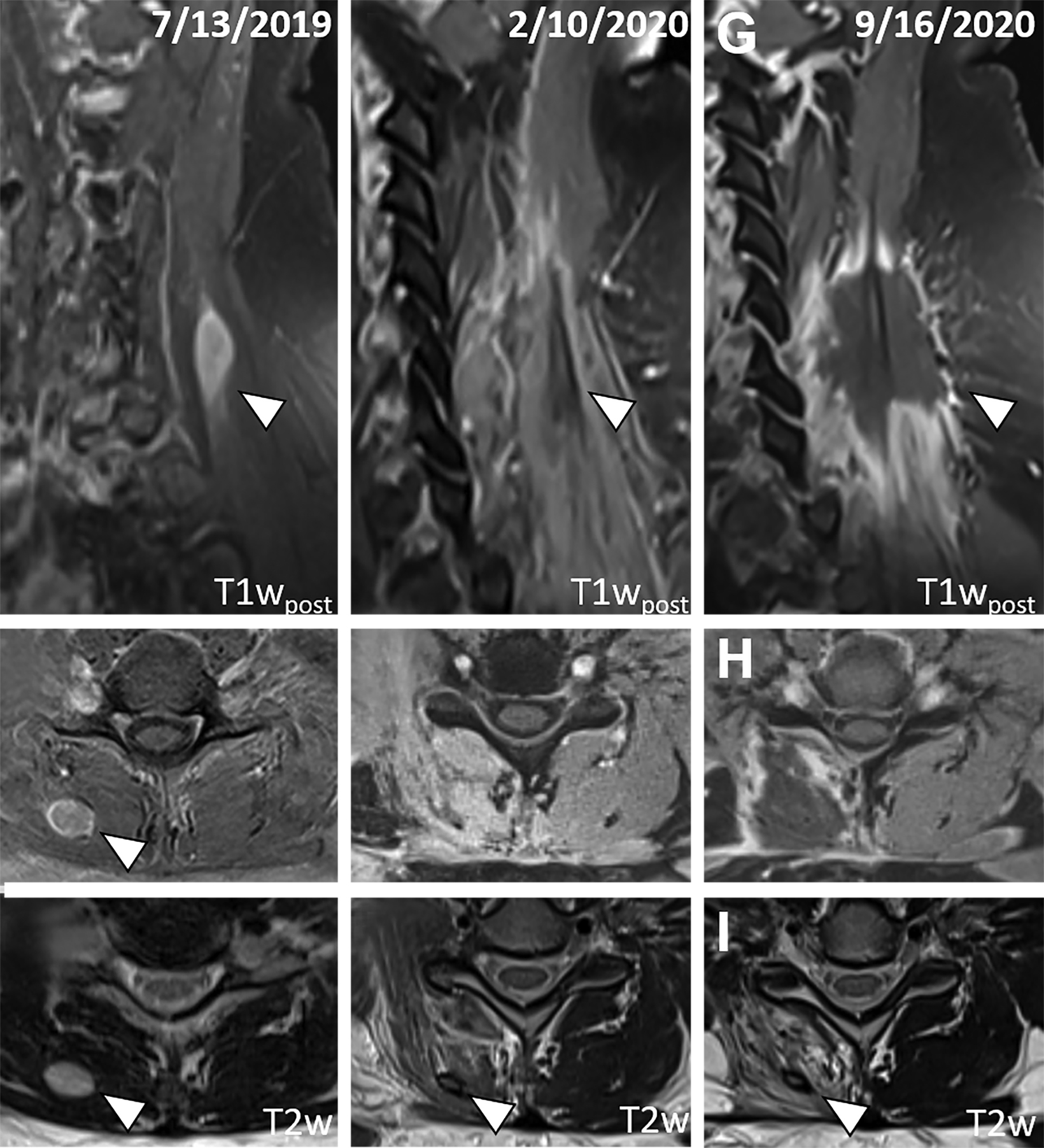
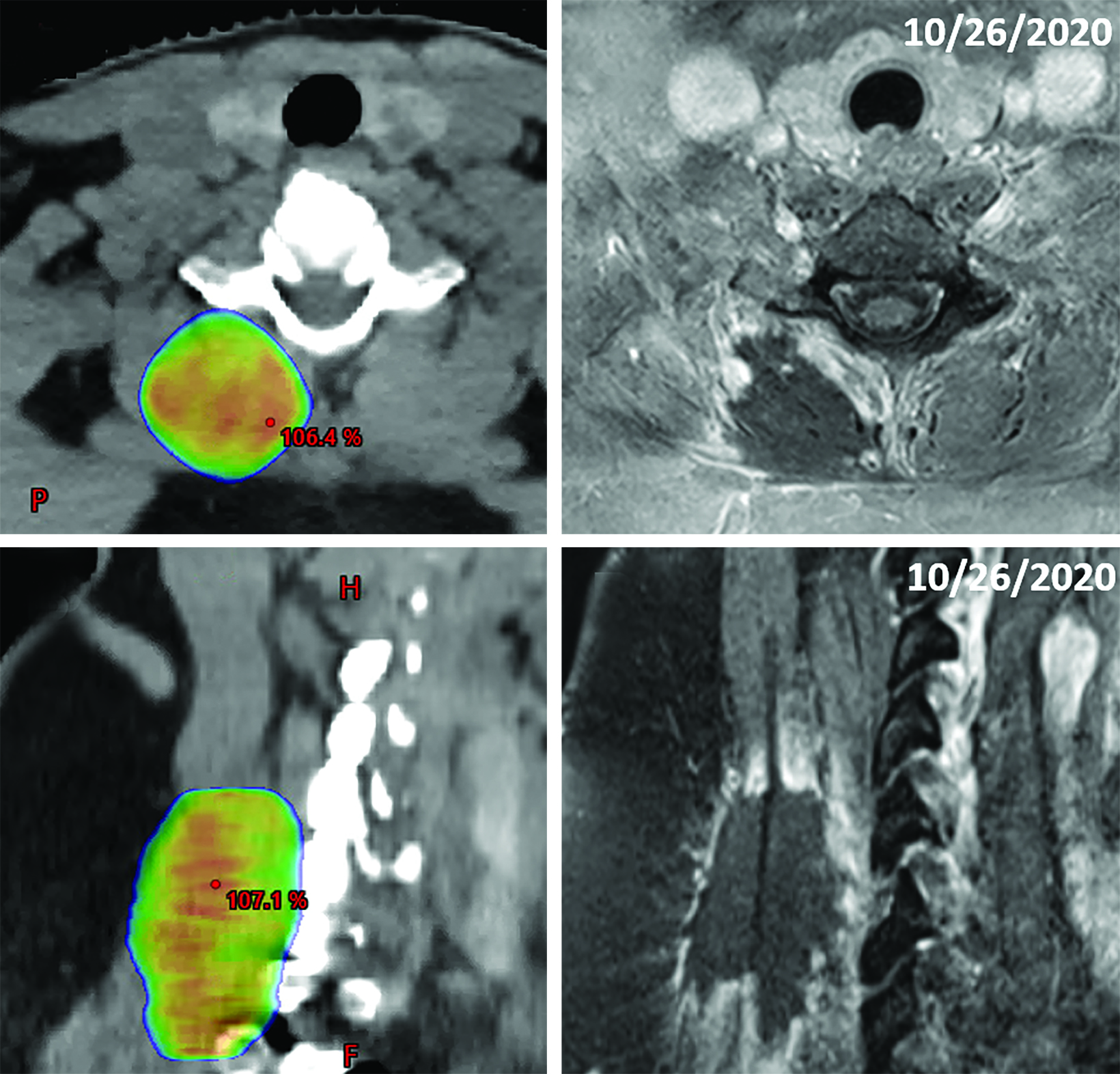
Following nephrectomy, systemic therapy was initiated with nivolumab. Within 8 months, she developed a C7 paravertebral 2.4 x 0.8-cm soft-tissue homogeneously enhancing deposit suspicious for metastasis (Figure 2A-C). Given disease progression, the patient was switched to combination pembrolizumab (200 mg infusion, 3 times weekly) and axitinib (5 mg, twice daily) 6 weeks after SBRT. The C7 lesion received SBRT to 40 Gy in 5 fractions, every other day. Radiation dose distributions are shown in Figure 3. She simultaneously had a lumbar spine lesion, which received 35 Gy in 5 fractions to comply with dose constraints in the pelvis and abdomen. Within several weeks of therapy, she reported neck stiffness. MRI of the cervical spine 8 weeks later showed no residual enhancement, indicating desirable treatment response. Twelve weeks later, she reported ongoing weakness in her neck, and difficulty holding up her head.
Five months following radiation, she reported intermittent neck pain. MRI of the cervical spine showed no nodular enhancement to suggest recurrence (Figure 2D and 2E). There was only mild, ill-defined right paraspinal muscle enhancement correlating with the radiation treatment field centered around a subcentimeter nonenhancing treated lesion with a T2 hypointense rim (Figure 2F). These findings suggested inflammation/myositis and were favored to be post-treatment changes.6,7
Fourteen months following RT, the neck pain/weakness worsened, and imaging revealed central hypoenhancement surrounded by a peripheral rim of irregular, patchy and “star-like” enhancement corresponding to the radiation field (Figure 2G and 2H). An axial T2-weighted image showed T2 hyperintensity of the right paravertebral muscles around an unchanged nonenhancing treated lesion (Figure 2I). This suggested myonecrosis within the treatment area.8-10 Four-week follow-up MRI showed the lesion was unchanged, signifying myonecrosis (Figure 3A-D).
After symptoms largely resolved, follow-up revealed a left C3 metastatic lesion that extended to the left deltoid muscle. After discussing treatment options, the patient opted for radiation therapy and received SBRT (40 Gy, 5 fractions) concurrent with systemic therapy to the lesion in February 2021. She now has radiographically stable radiation myonecrosis in the site at C7 and has only minimal pain with excessive movement, but has no further development of any other site of radiation myonecrosis.
Discussion
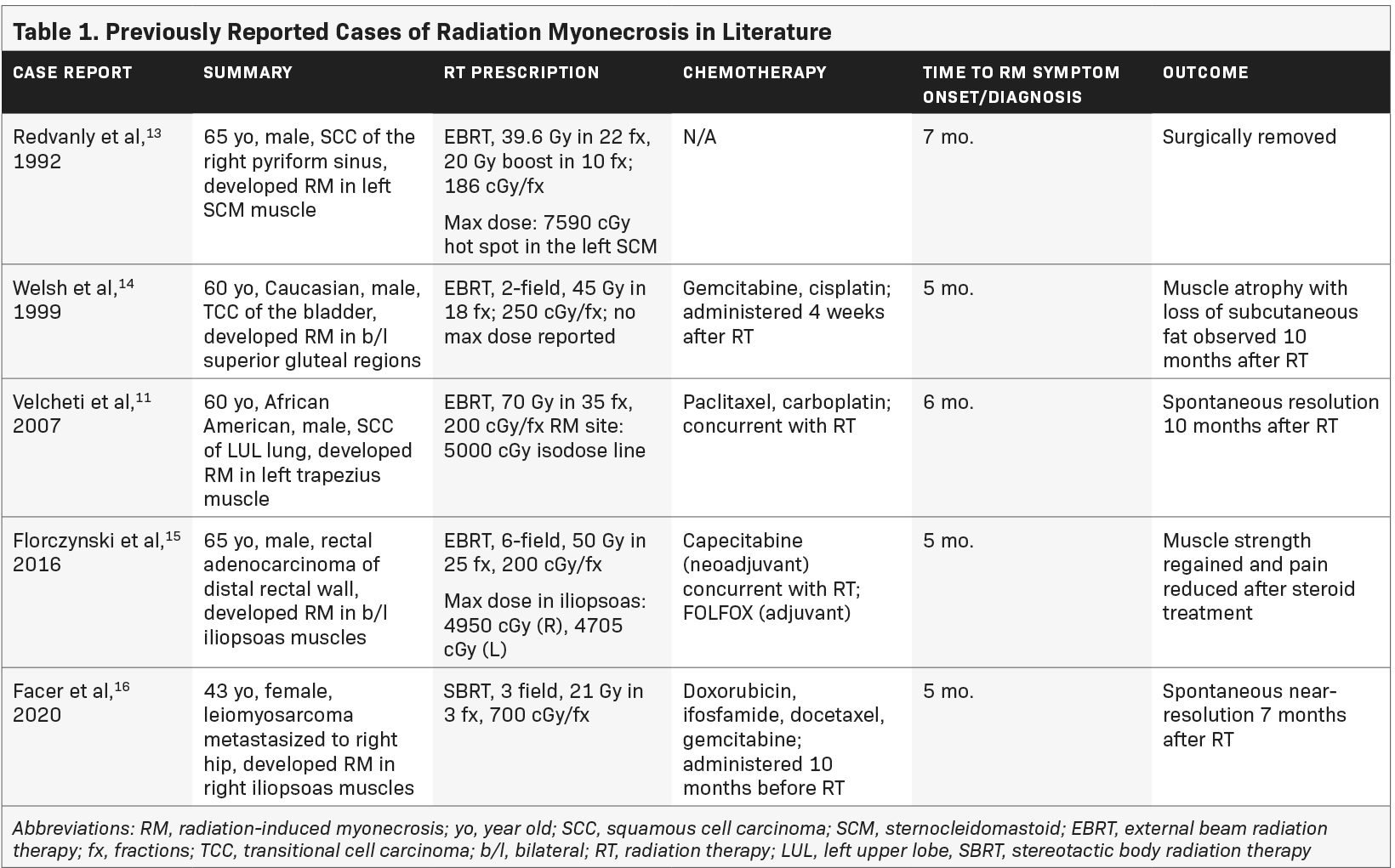
Little is known about radiation-induced myonecrosis. It is believed to be caused by vascular insufficiency from proliferation of collagen resulting in ischemia.11 Use of concurrent chemotherapy along with radiation increases the risk of acute and delayed radiation-related side effects.12 Just 5 other case reports are published regarding myonecrosis, none of which are associated with RCC. Table 1 summarizes the previous cases reported in the literature.
In 1992, Redvanly et al reported myonecrosis in the left sternocleidomastoid muscle following radiation for a squamous cell carcinoma of the pyriform sinus. The maximum dose point dose to the sternocleidomastoid was 7590 cGy. Necrotic muscle was surgically removed, and histology revealed myonecrosis. We concluded that this is a case of radiation-induced myonecrosis.13
In 1999, Welsh et al described radiation myonecrosis in a patient who received 45 Gy in 18 fractions to the inferior pelvis for a parasacral mass from bladder cancer. He received adjuvant gemcitabine and cisplatin. Five months following RT, he developed gluteal pain bilaterally and MRI revealed a band-like pattern of edema on T2-weighted images. He was managed on NSAIDs and a short course of prednisone. Ten months following RT, he had atrophy and loss of the overlying subcutaneous fat.14
In 2007, Velcheti et al reported radiation myonecrosis of the left trapezius in a patient receiving 70 Gy in 35 fractions to his left upper lobe; he was on concurrent paclitaxel/carboplatin. Six months following radiation, the patient developed pain in his upper back, corresponding to the area that received at least 50 Gy. Biopsy revealed skeletal muscle with necrosis. Four months later, an MRI was obtained and findings were consistent with radiation myonecrosis.11
In 2016, Florczynski et al published a case of severe myositis of the hip flexors in a patient on concurrent capecitabine for rectal cancer receiving adjuvant FOLFOX. Five months following radiation, he developed bilateral weakness of the iliopsoas muscles. Authors concluded from clinical and radiographic findings that this was radiation myonecrosis induced through radiation recall after starting FOLFOX following the completion of radiation.15
In 2020, Facer et al described a case of radiation myonecrosis in the iliopsoas muscle following SBRT for leiomyosarcoma in the right iliac bone. This patient completed treatment with docetaxel and gemcitabine and then developed pain in the right hip associated with a lesion that was treated with SBRT to the iliac bone (21 Gy in 3 fractions). Five months later, the patient was diagnosed with radiation-induced myonecrosis by MRI. Similar to other cases, the MRI showed band-like abnormal signal intensity in the area overlapping radiation. Follow-up MRI at 7 months showed persistent radiographic changes.16
Our report is the first radiation myonecrosis case resulting from radiation therapy for RCC. Clinically, the patient experienced general stiffness and weakness in the cervical lesion and was in moderate but tolerable pain. She was instructed to use heat, ice, and over-the-counter medicine as home treatments to relieve symptoms. As shown in Figure 3, the radiation treatment area directly overlaps with the site of myonecrosis. Our case demonstrates that radiation myonecrosis occurred with a dose fractionation of 40 Gy in 5 fractions (the bioequivalent dose would be ~131 Gy, assuming α/β ~3.5 for muscle).17 This dose is well above the reported threshold of 55 Gy, which is known to cause late muscle morbidity after radiation therapy, particularly in sarcomas.18
Our patient was not treated with conventional radiation. Instead, similar to the myonecrosis case presented by Facer et al in 2020, our patient received SBRT, which has a much higher dose per fraction (800 cGy). Previous reports have associated spinal SBRT with an increased risk of radiation-induced myositis, which could further cause myonecrosis.16,19 These recorded associations between SBRT and radiation-induced myopathies suggest that our use of SBRT may have contributed to our patient’s myonecrosis.
Interestingly, our patient received no chemotherapies that were reported in the previous 5 historical radiation myonecrosis cases. Rather, she underwent systemic therapy using pembrolizumab/axitinib, a combination anti-PD-1/L1 immunotherapy with a VEGF/ VEGFR inhibitor (a preferred regimen).20 While there are no reports of radiation-induced myonecrosis in patients on concurrent pembrolizumab/axitinib, it is possible that these agents and high-dose radiation contributed to the development of her radiation myonecrosis. It is also worth noting that in the previously reported radiation myonecrosis cases, all patients received systemic therapies, which suggests that general caution should be taken in treating with concurrent SBRT and systemic therapies. Case reports in the past have suggested that one of our patient’s immunotherapies, pembrolizumab, could induce necrotizing myositis in patients.21-23 It is therefore possible that pembrolizumab secondarily influenced the formation of radiation myonecrosis. Furthermore, the VEGF inhibition effect of axitinib might exacerbate toxicity by decreasing angiogenesis in the lesion. Little is reported on concurrent axitinibradiation therapy, but ongoing clinical trials on the combination therapy do not report any related adverse effects either.24
Our case features an atypical course of radiation myonecrosis. In the 5 reported cases, imaging diagnosis could be made approximately 5 to 7 months after radiation therapy, but our case was diagnosed radiographically at 14 months. This suggests variability in the course of disease for radiation myonecrosis and should be considered for future diagnoses. Interestingly, our patient developed myonecrosis overlapping the 40 Gy in 5 fractions in her cervical spine but did not develop this phenomenon in her lower spine where she was simultaneously treated to 35 Gy in 5 fractions. She did not have other adverse side effects after either of the other 2 treatments.
From the radiographic findings and clinical presentations in this case, we assumed a true radiographic diagnosis of radiation-induced myonecrosis. The differential diagnosis included tumor recurrence, infection, and radiation recall myositis. Stability of radiographic presentation over follow-up MRIs rules out tumor progression and infection was ruled out from clinical and laboratory findings. This case differentiates itself from radiation recall myositis in several ways. Radiation recall myositis is most likely triggered by gemcitabine and sometimes triggered by other chemotherapeutics.25 The mechanisms of action of these agents are either related to DNA damage and synthesis or microtubule function, which differ from our patient’s therapy of pembrolizumab and axitinib. Pembrolizumab is associated with cutaneous and pulmonary radiation recall reactions,26,27 but not with radiation recall myositis. Axitinib has no reports of radiation recall reactions. Therefore, it is unlikely that the patient’s symptoms were caused by radiation recall myositis.
Conclusion
To our knowledge, only 5 other reported cases describe this postradiation phenomenon. Our case shows both similarities and differences to the previously reported cases in terms of symptom presentation, disease timeline and treatments received. In this modern era, it is important to consider the possible effects of immunotherapy and systemic therapies in general with radiation therapy. We add this to the published collection of radiation-induced myonecrosis cases and caution experts on this rare but serious potential side effect of radiation therapy.
References
- Padala SA, Barsouk A, Thandra KC, et al. Epidemiology of renal cell carcinoma. World J Oncol. 2020;11(3):79-87. doi:10.14740/wjon1279
- Mohamed AH, Mohamud HA. Renal cell carcinoma with rhabdoid features: a rare aggressive and fatal variant. Urol Case Rep. 2020;32:101244. doi:10.1016/j. eucr.2020.101244
- Deschavanne PJ, Fertil B. A review of human cell radiosensitivity in vitro. Int J Radiat Oncol Biol Phys. 1996;34(1):251-266. doi:10.1016/0360-3016(95)02029-2
- Blanco AI, Teh BS, Amato RJ. Role of radiation therapy in the management of renal cell cancer. Cancers (Basel). 2011;3(4):4010-4023. doi:10.3390/cancers3044010
- Rühle A, Andratschke N, Siva S, Guckenberger M. Is there a role for stereotactic radiotherapy in the treatment of renal cell carcinoma? Clin Transl Radiat Oncol. 2019;18:104-112. doi:10.1016/j.ctro.2019.04.012
- Schulze M, Kötter I, Ernemann U, et al. MRI findings in inflammatory muscle diseases and their noninflammatory mimics. Am J Roentgenol. 2009;192(6):1708-1716. doi:10.2214/AJR.08.1764
- Garner HW, Kransdorf MJ, Bancroft LW, Peterson JJ, Berquist TH, Murphey MD. Benign and malignant soft-tissue tumors: posttreatment MR imaging. RadioGraphics. 2009;29(1):119-134. doi:10.1148/rg.291085131
- Becker M, Schroth G, Zbären P, et al. Long-term changes induced by high-dose irradiation of the head and neck region: imaging findings. RadioGraphics. 1997;17(1):5-26. doi:10.1148/radiographics.17.1.9017796
- Smitaman E, Flores DV, Mejía Gómez C, Pathria MN. MR imaging of atraumatic muscle disorders. RadioGraphics. 2018;38(2):500-522. doi:10.1148/rg.2017170112
- Theodorou DJ, Theodorou SJ, Kakitsubata Y. Skeletal muscle disease: patterns of MRI appearances. Br J Radiol. 2012;85(1020):e1298-e1308. doi:10.1259/bjr/14063641
- Velcheti V, Gilstrap E, Bradley J, Govindan R. Radiation-induced myonecrosis presenting as a subcutaneous mass after combined modality therapy for non-small cell lung cancer. J Thorac Oncol. 2007;2(9):875-876. doi:10.1097/JTO.0b013e31811f3a91
- Byhardt RW, Scott C, Sause WT, et al. Response, toxicity, failure patterns, and survival in five Radiation Therapy Oncology Group (RTOG) trials of sequential and/or concurrent chemotherapy and radiotherapy for locally advanced non-small-cell carcinoma of the lung. Int J Radiat Oncol Biol Phys. 1998;42(3):469-478. doi:10.1016/s0360-3016(98)00251-x
- Redvanly RD, Hudgins PA, Gussack GS, Lewis M, Crocker IR. CT of muscle necrosis following radiation therapy in a patient with head and neck malignancy. Am J Neuroradiol. 1992;13(1):220-222.
- Welsh JS, Torre TG, DeWeese TL, O’Reilly S. Radiation myositis. Ann Oncol. 1999;10(9):1105-1108. doi:10.1023/a:1008365221440
- Florczynski MM, Sanatani MS, Mai L, et al. Severe myositis of the hip flexors after pre-operative chemoradiation therapy for locally advanced rectal cancer: case report. BMC Cancer. 2016;16:243. doi:10.1186/s12885-016-2269-2
- Facer BD, Dutta SW, Showalter TN. Stereotactic body radiation therapy induced myonecrosis in a patient with prior gemcitabine administered for leiomyosarcoma. J Radiosurg SBRT. 2020;7(1):77-80.
- Kotha VS, Rewari A, Lakhiani C, et al. Radiation oncology applications in plastic and reconstructive surgery: a nonsystematic review of concepts and prin- ciples. Plast Reconstr Surg. 2021;147(2):314e-324e. doi:10.1097/PRS.0000000000007582
- Karasek K, Constine LS, Rosier R. Sarcoma therapy: functional outcome and relationship to treatment parameters. Int J Radiat Oncol Biol Phys. 1992;24(4):651-656. doi:10.1016/0360-3016(92)90710-y
- Lockney DT, Jia AY, Lis E, et al. Myositis following spine radiosurgery for metastatic disease: a case series. J Neurosurg Spine. 2018;28(4):416-421. doi:10.3171/2017.8.SPINE17162
- Rini BI, Plimack ER, Stus V, et al. Pembrolizumab (pembro) plus axitinib (axi) versus sunitinib as first-line therapy for advanced clear cell renal cell carci- noma (ccRCC): results from 42-month follow-up of KEYNOTE-426. JCO. 2021;39(15_suppl):4500-4500. doi:10.1200/JCO.2021.39.15_suppl.4500
- Claus J, Van Den Bergh A, Verbeek S, Wauters E, Nackaerts K. Pembrolizumab-induced necrotizing myositis in a patient with metastatic non-small-cell lung cancer: a case report. Lung Cancer Manag. 2019;8(2):LMT10. doi:10.2217/lmt-2018-0017
- Haddox CL, Shenoy N, Shah KK, et al. Pembrolizumab induced bulbar myopathy and respiratory failure with necrotizing myositis of the diaphragm. Ann Oncol. 2017;28(3):673-675. doi:10.1093/annonc/mdw655
- Vallet H, Gaillet A, Weiss N, et al. Pembrolizumab-induced necrotic myositis in a patient with metastatic melanoma. Ann Oncol. 2016;27(7):1352-1353. doi:10.1093/annonc/mdw126
- Yang KL, Chi MS, Ko HL, et al. Axitinib in combination with radiotherapy for advanced hepatocellular carcinoma: a phase I clinical trial. Radiat Oncol. 2021;16(1):18. doi:10.1186/s13014-020-01742-w
- Maeng CH, Park JS, Lee SA, et al. Radiation recall phenomenon presenting as myositis triggered by carboplatin plus paclitaxel and related literature review. J Cancer Res Ther. 2014;10(4):1093-1097. doi:10.4103/0973-1482.146090
- Wang YY, Tian XC, Zhu L, Bai XH, Zhao R. Concomitant radiation recall dermatitis and radiation recall pneumonitis induced by pembrolizumab. J Thorac Oncol. 2020;15(10):e160-e162. doi:10.1016/j.jtho.2020.05.014
- Itamura H, Ohguri T, Yahara K, et al. Pembrolizumab-induced radiation recall pneumonitis after the resolution of typical asymptomatic radiation pneumonitis. J UOEH. 2020;42(3):261-266. doi:10.7888/juoeh.42.261
Citation
Su Y, Dastgheyb S, Balzer-Haas N, Song JW, Jones J. Teaching Case: Radiation Myonecrosis Following Stereotactic Body Radiation Therapy in Metastatic Renal Cell Carcinoma. Appl Radiat Oncol. 2022;(4):26-31.
December 23, 2022
