Companies Sign Distribution Agreement for Prostate Cancer Diagnostic
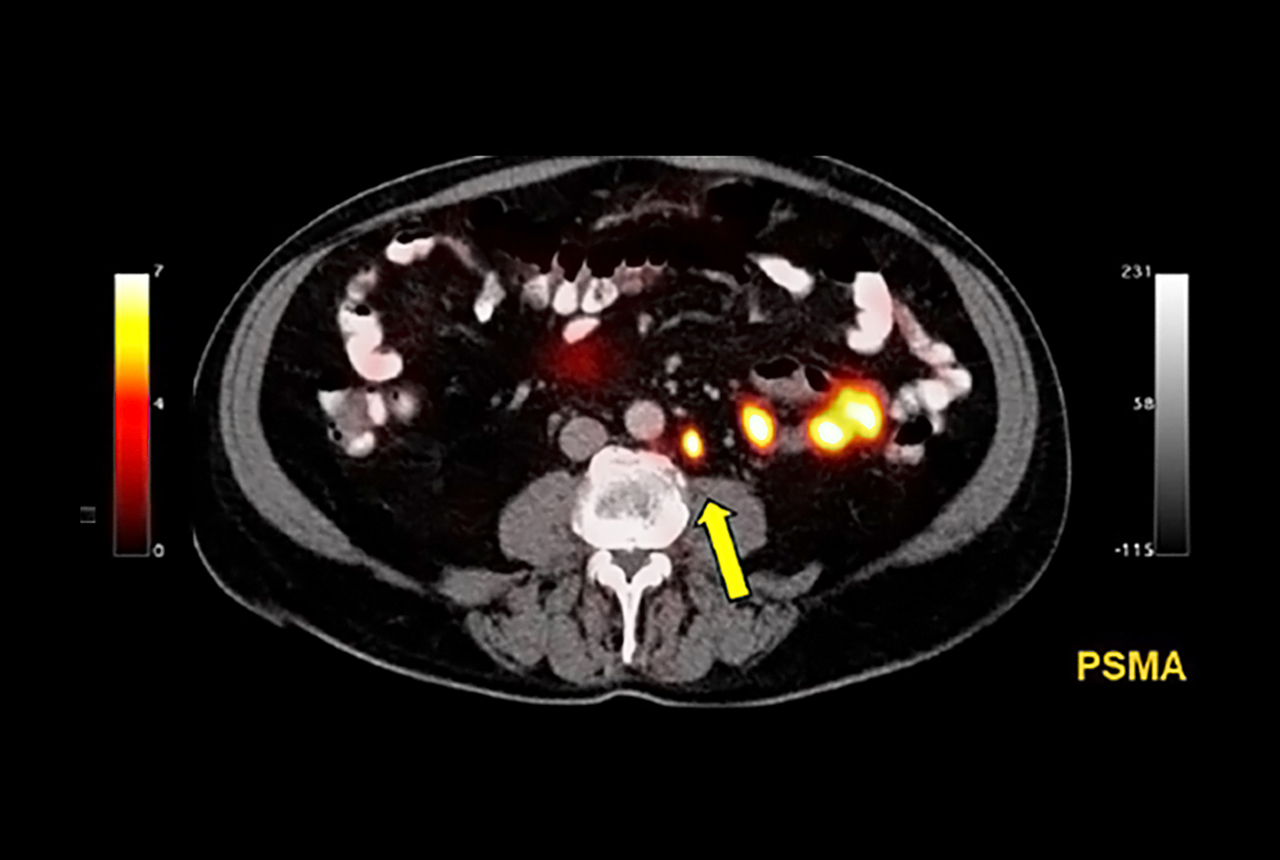 Eckert & Ziegler signed an exclusive distribution agreement with Telix Pharmaceuticals for Illuccix, a kit for the preparation of Ga-68 PSMA-11 injection, in Germany. Illuccix is a preparation for imaging prostate cancer with positron emission tomography (PET), currently under review for regulatory approval in multiple markets worldwide, including Germany. Illuccix (TLX591-CDx) for prostate cancer imaging, has been accepted for filing by the US FDA and is under priority evaluation by the Australian Therapeutic Goods Administration (TGA). Telix is also progressing marketing authorization applications for Illuccix in the European Union and Canada.
Eckert & Ziegler signed an exclusive distribution agreement with Telix Pharmaceuticals for Illuccix, a kit for the preparation of Ga-68 PSMA-11 injection, in Germany. Illuccix is a preparation for imaging prostate cancer with positron emission tomography (PET), currently under review for regulatory approval in multiple markets worldwide, including Germany. Illuccix (TLX591-CDx) for prostate cancer imaging, has been accepted for filing by the US FDA and is under priority evaluation by the Australian Therapeutic Goods Administration (TGA). Telix is also progressing marketing authorization applications for Illuccix in the European Union and Canada.
"Illuccix is anticipated to be one of the most important imaging products for prostate cancer. Widespread approval of a preparation for the diagnosis of prostate cancer is urgently needed and we are pleased to have Telix, a pioneer in bringing this drug to market, as a partner," explained Dr. Harald Hasselmann, member of the Executive Board of Eckert & Ziegler AG and responsible for the Medical segment. "Together with our other products, we will be able to offer nuclear medicine practices and clinics in Germany a fully comprehensive product portfolio for the production of Ga-68 PSMA, once approval has been attained."
We are pleased to have entered this commercial distribution agreement with Eckert & Ziegler so that, subject to German regulatory approval, we will together be able to deliver a commercial product to German patients living with prostate cancer as efficiently as possible. Partnering with such a capable and patient-centric leader in nuclear medicine uniquely aligns with Telix's mission of helping patients with cancer live longer, better quality lives", explained Telix Chief Executive Officer Dr. Christian Behrenbruch.
Illuccix is offered as a so-called kit preparation for the diagnosis of prostate cancer. For this purpose, Illuccix enables PSMA-11 to be labelled with the radionuclide Ga-68 directly before injection by medical personnel. After preparing the radiopharmaceutical and injecting it into the patient, tumors that show the so-called prostate-specific membrane antigen can be localized by PET.