FDA Fast Tracks T-Cell Agonist for PD-1 Resistant Colorectal Cancer Tumors
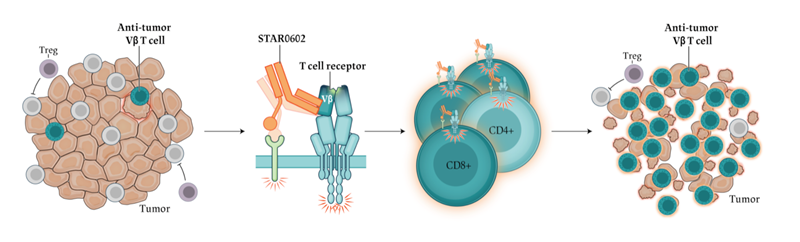 Marengo Therapeutics, Inc. announced that the US FDA has granted Fast Track designation to invikafusp alfa (STAR0602), Marengo's first-in-class selective dual T cell agonist being studied as a potential new treatment for advanced colorectal cancer with TMB-H. Fast Track designation is designed to facilitate the development and expedite the review of therapies intended to treat serious or life-threatening conditions with unmet medical needs.
Marengo Therapeutics, Inc. announced that the US FDA has granted Fast Track designation to invikafusp alfa (STAR0602), Marengo's first-in-class selective dual T cell agonist being studied as a potential new treatment for advanced colorectal cancer with TMB-H. Fast Track designation is designed to facilitate the development and expedite the review of therapies intended to treat serious or life-threatening conditions with unmet medical needs.
"The FDA's Fast Track designation is an important milestone for the STAR0602 program and further positions our unique selective dual T cell agonist platform as a promising solution to address key challenges that perpetuate significant unmet needs in oncology," said Zhen Su, MD, MBA, Chief Executive Officer of Marengo Therapeutics. "This recognition specifically validates the promise of STAR0602 as a novel treatment option for patients with TMB-H metastatic colorectal cancer, which is insensitive to PD-1 treatment."
The FDA's decision is informed by the encouraging results from Marengo's first-in-human Phase 1 clinical study of invikafusp alfa in heavily pretreated cancer patients, which were recently presented during a plenary oral session at the 2024 SITC Annual Meeting and an oral presentation at the 2024 ESMO Immuno-Oncology Congress. The data reinforce invikafusp alfa's anti-tumor activity and favorable safety profile.
"Marengo's selective Vβ T cell activation approach targeting specific T cell subsets enriched in Tumor-infiltrating lymphocytes to enhance anti-tumor activity is unique and highly promising," said Bruce Chabner, MD, Clinical Director Emeritus for the Massachusetts General Hospital Cancer Center and Professor of Medicine at Harvard Medical School. "The Phase 2 clinical investigation of invikafusp alfa is ongoing and this novel treatment could lead to a new class of therapeutics for tumor types that are PD-1 insensitive or resistant, especially in colorectal cancer where current treatment options remain limited."
