POSLUMA Shows High Detection Rates in African-American Men with Prostate Cancer
Images
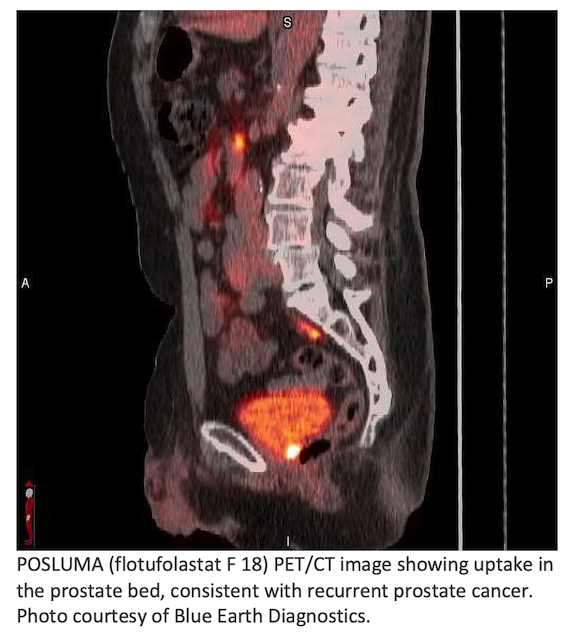
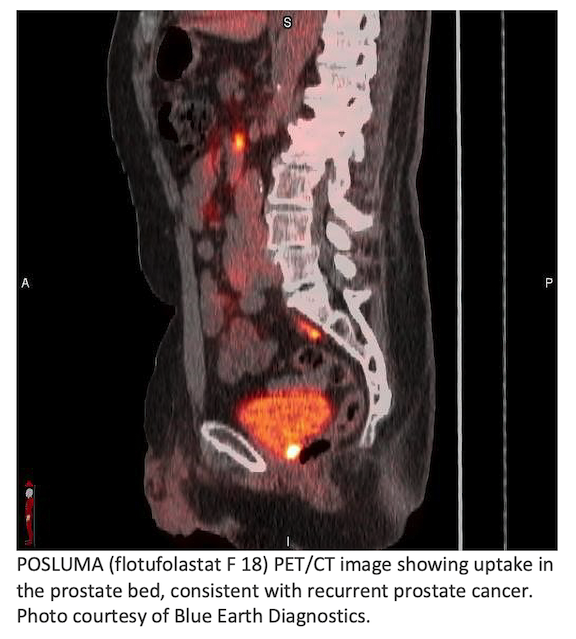 Blue Earth Diagnostics announced post-hoc results of the Phase 3 SPOTLIGHT study assessing FDA-approved POSLUMA (flotufolastat F 18) injection in patients with recurrent prostate cancer. 3,4 Results demonstrated consistent performance between African-Americans and other patient groups.1 The study achieved 17% African American enrollment, more than twice the average for oncology clinical trials.2 Blue Earth Diagnostics is a Bracco company and recognized leader in the development and commercialization of innovative PET radiopharmaceuticals.
Blue Earth Diagnostics announced post-hoc results of the Phase 3 SPOTLIGHT study assessing FDA-approved POSLUMA (flotufolastat F 18) injection in patients with recurrent prostate cancer. 3,4 Results demonstrated consistent performance between African-Americans and other patient groups.1 The study achieved 17% African American enrollment, more than twice the average for oncology clinical trials.2 Blue Earth Diagnostics is a Bracco company and recognized leader in the development and commercialization of innovative PET radiopharmaceuticals.
Based on the high mortality and prevalence of prostate cancer in African American men, a sub-analysis was conducted to evaluate the performance of POSLUMA and the rate of enrollment for African American men in the trial. Results showed that the detection rate was high among African American patients, with 93% found to have a positive POSLUMA scan, consistent with the 87% detection rate for all other patients in the study.1 The 17% participation of African American men in the SPOTLIGHT study was twice the enrollment typically reported in other types of oncology clinical trials (8.5%).1,2 The manuscript, “18F-Flotufolastat Positron Emission Tomography in African American Patients with Suspected Prostate Cancer Recurrence: Findings from the Phase 3 SPOTLIGHT Study” has been published online in the journal Advances in Radiation Oncology, and will appear in an upcoming print issue.
POSLUMA is approved in the United States for positron emission tomography (PET) of prostate-specific membrane antigen (PSMA) positive lesions in men with prostate cancer with suspected metastasis who are candidates for initial definitive therapy or with suspected recurrence based on elevated serum prostate-specific antigen (PSA) level.
“Prostate cancer is the most commonly diagnosed cancer among African American men, who are twice more likely to die from the disease than White men,”5-7 said Soroush Rais-Bahrami, MD, Department of Urology, University of Alabama at Birmingham Heersink School of Medicine, Birmingham, Ala. “It is encouraging that African American enrollment in the SPOTLIGHT trial closely aligns with their 14% representation of the U.S. population8, because results from oncology trials with low diversity populations are less useful for clinical decision-making and can contribute to racial disparities in cancer outcomes.”
The U.S. Food and Drug Administration (FDA) has issued draft guidance on clinical trial diversity and organizations such as the American Society of Clinical Oncology (ASCO) have called out the need for enriched diversity in oncology clinical trial participation.9,10
“Beyond validating the diagnostic performance of POSLUMA in African American men, findings from the SPOTLIGHT study provide useful considerations for planning future clinical trials, to facilitate patient enrollment and achieve clinical trial diversity,” said Marco Campione, Chief Executive Officer, Blue Earth Diagnostics. “The results achieved in African American men were derived from clinical sites across the United States, and further support the broad applicability of POSLUMA for its indicated use across the U.S. population as a whole.”
FDA-approved POSLUMA represents a newer class of high-affinity PSMA-targeted PET radiopharmaceuticals based on novel radiohybrid technology and leverages the high image quality of 18F-labeled PSMA PET imaging to help facilitate detection of prostate cancer. It can provide clinically valuable information to guide patient management based on its high-affinity PSMA binding, low urinary uptake and positive performance at very low PSA levels. POSLUMA is included in nationally recognized clinical oncology guidelines for prostate cancer, covered by the vast majority of insurance plans and is readily available for patients across the country through the network of Blue Earth Diagnostics’ commercial U.S. manufacturer and distributor, PETNET Solutions Inc, A Siemens Healthineers Company.
References
1. Rais-Bahrami S, Fleming M, Gartrell B, et al. 18F-Flotufolastat Positron Emission Tomography in African American Patients with Suspected Prostate Cancer Recurrence: Findings from the Phase 3 SPOTLIGHT Study. Adv Clin Onc 2024; doi 10.1016/j.adro.2024.101571
2. Bebi T, Horovitz R, Blum K, et al. How granularity of data matters in understanding and accelerating racial diversity in U.S. clinical trials. J Clin Oncol2022;40 (Suppl 28):88.
3. Jani AB, Ravizzini G, Gartrell BA, et al. Diagnostic performance and safety of 18F-rhPSMA-7.3 PET in men with suspected prostate cancer recurrence: Results from a phase 3, prospective, multicenter study (SPOTLIGHT) J Urol2023;210:299-311.
4. FDA. Highlights of prescribing information: Posluma (flotufolastat f 18) injection: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/216023s000lbl.pdf; 2023.
5. Giaquinto AN, Miller KD, Tossas KY, et al. Cancer statistics for African American/Black people 2022. CA Cancer J Clin 2022;72:202‐229.
6. Frego N, Labban M, Stone BV, et al. Effect of type of definitive treatment on race‐based differences in prostate cancer-specific survival. Prostate 2023;83:1099‐1111.
7. Rais‐Bahrami S, Zhu Y. Disparities in prostate cancer diagnosis and management: Recognizing that disparities exist at all junctures along the prostate cancer journey. Prostate cancer and prostatic diseases 2023;26:441‐442.
8. Facts About the U.S. Black Population. Pew Research Center. Fact Sheet. January 14, 2024. https://www.pewresearch.org/social-trends/fact-sheet/facts-about-the-us-black-population/
9. FDA. Diversity Action Plans to Improve Enrollment of Participants from Underrepresented Populations in Clinical Studies https://www.fda.gov/media/179593/download; 2024.
10. Oyer RA, Hurley P, Boehmer L, et al. Increasing racial and ethnic diversity in cancer clinical trials: An American Society of Clinical Oncology and Association of Community Cancer Centers joint research statement. Journal of Clinical Oncology 2022;40:2163-2171.
