SNMMI 2022 Abstract of the Year: Radionuclide Treatment for Advanced-Stage Neuroendocrine Tumors
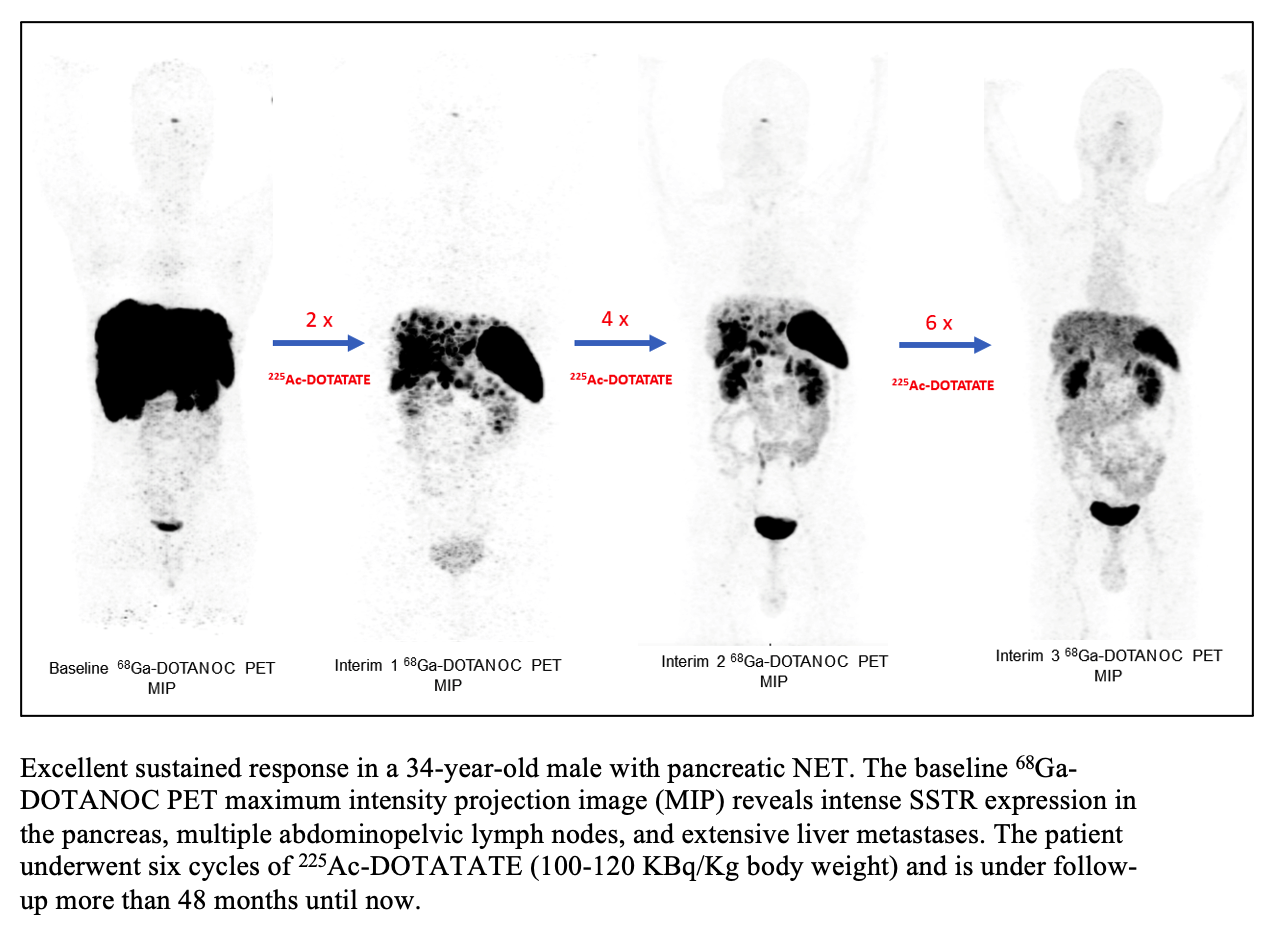 The 2022 SNMMI Henry N. Wagner, Jr., Abstract of the Year was given to research presented on a targeted radionuclide alpha therapy,225Ac-DOTATATE, that has been shown to have long-term anti-tumor effects in patients with advanced-stage gastroenteropancreatic neuroendocrine tumors (GEP-NETs). Results from the Phase II study showed promising survival rates, high response rates and an acceptable toxicity profile, making225Ac-DOTATATE a potential treatment option for patients who have exhausted other forms of therapy.
The 2022 SNMMI Henry N. Wagner, Jr., Abstract of the Year was given to research presented on a targeted radionuclide alpha therapy,225Ac-DOTATATE, that has been shown to have long-term anti-tumor effects in patients with advanced-stage gastroenteropancreatic neuroendocrine tumors (GEP-NETs). Results from the Phase II study showed promising survival rates, high response rates and an acceptable toxicity profile, making225Ac-DOTATATE a potential treatment option for patients who have exhausted other forms of therapy.
GEP-NETs are rare malignancies that arise from neuroendocrine cells and can be present all along the gastroinstesinal tract. While patients with early-stage GEP-NETs can undergo surgery to cure the disease, the majority of patients are diagnosed with metastatic disease, making systemic treatment—such as targeted radionuclide therapy—their only option.177Lu-DOTATATE has been proven to be an effective targeted beta radionuclide therapy; little evidence, however, is available on targeted alpha radionuclide therapies for GEP-NETs.
In the study, researchers aimed to evaluate the long-term efficacy, survival outcomes, and safety of the targeted radionuclide alpha therapy225Ac-DOTATATE in GEP-NET patients. Eighty-three GEP-NET patients (including 56 patients with prior177Lu-DOTATATE treatment and 27177Lu-DOTATATE-naïve patients) received systemic treatment with225Ac-DOTATATE intravenously at eight weekly intervals.
Hematologic, kidney and liver function tests were performed before and after each cycle. Tumors were measured using RECIST 1.1 criteria at baseline and after two cycle intervals. Overall survival, radiographic progression-free survival, objective tumor response, and treatment-related adverse events were assessed.
After the treatment course, two patients (2.7%) had complete response, 32 (43.2%) had a partial response, 25 (34%) had stable disease, and 15 (20%) had progressive disease. Minimal toxicities were noted after treatment with225Ac-DOTATATE.
“225Ac-DOTATATE is a promising therapy option that adds a new dimension to the treatment of end-stage GEP-NETs, especially for patients who have tried all other standard therapy options,” said Chandrasekhar S. Bal, MD, DNB, DSc (HC), FAMS, FNASc, FASc, professor and head of the Department of Nuclear Medicine and PET at the All India Institute of Medical Science in New Delhi, India. “These results warrant a Phase III randomized control trial to assess the true efficacy of225Ac-DOTATATE versus177Lu-DOTATATE.”
“The results from this study not only emphasized the promise and success of targeted alpha therapies but also reflected growing global interest in these life-extending treatments,” said Heather Jacene, MD, SNMMI Scientific Program Committee chair. “We look forward to further research on this topic in the future.”