SNMMI ’21: PSMA-Targeted Radiotracer Pinpoints Metastatic Prostate Cancer
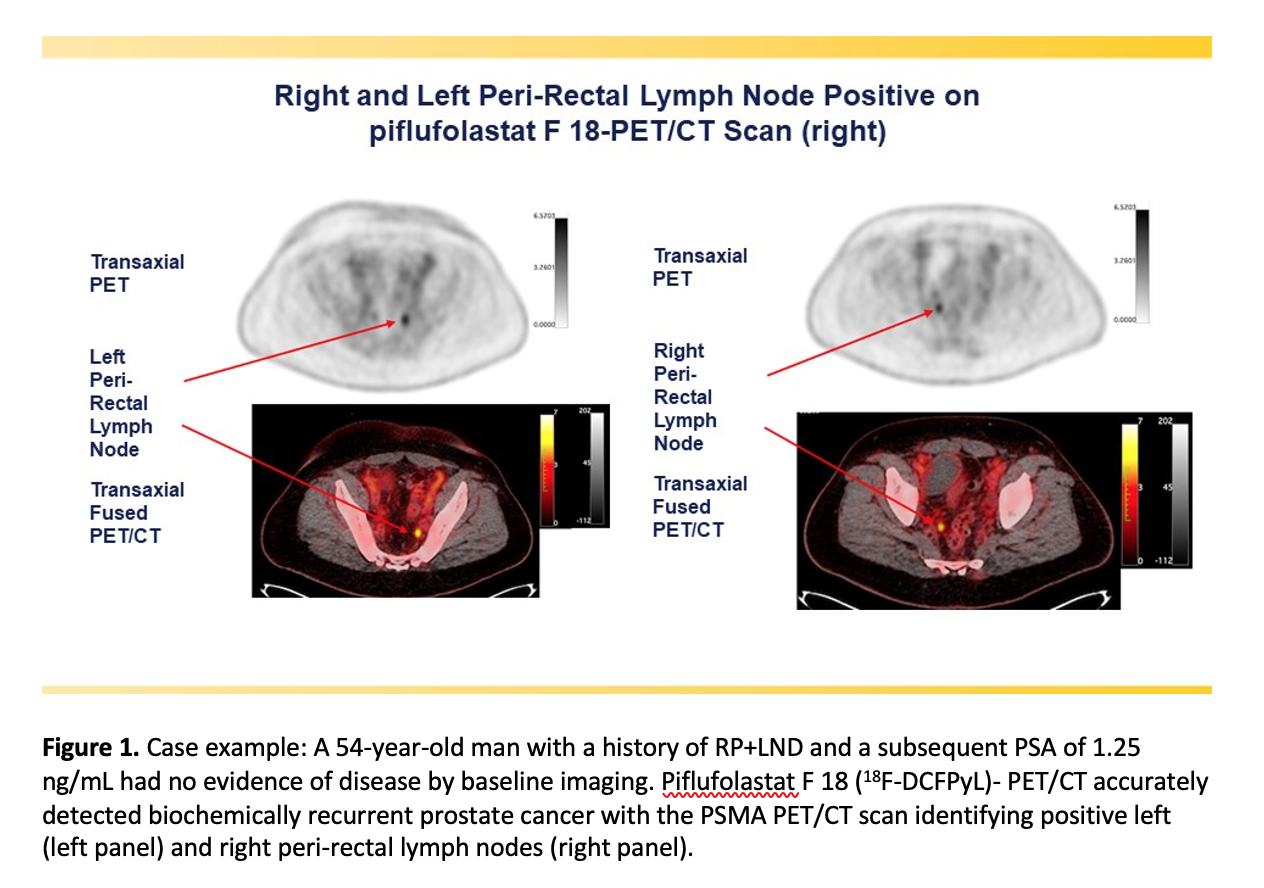 A phase III clinical trial has validated the effectiveness of the prostate-specific membrane antigen (PSMA)-targeted radiotracer18F-DCFPyL in detecting and localizing recurrent prostate cancer.
A phase III clinical trial has validated the effectiveness of the prostate-specific membrane antigen (PSMA)-targeted radiotracer18F-DCFPyL in detecting and localizing recurrent prostate cancer.
Approved by the U.S. Food and Drug Administration (FDA) last month, the radiotracer identified metastatic lesions with high positive predictive values regardless of anatomic region, adding to the evidence that PSMA-targeted radiotracers are the most sensitive and accurate agents for imaging prostate cancer. This study was presented at the Society of Nuclear Medicine and Molecular Imaging 2021 Annual Meeting.
In previous studies, the novel positron emission tomography (PET) imaging agent18F-DCFPyL was found to bind selectively with high affinity to PSMA. To demonstrate the diagnostic performance of18F-DCFPyL (now referred to as piflufolastat F-18) for regulatory approval, a prospective, multicenter study was conducted in 14 sites across the United States and Canada.
The study sought to determine the positive predictive value (the probability that patients with a positive screening test actually have the disease) and detection rate of18F-DCFPyL PET/computed tomography (CT) by anatomic region.18F-DCFPyL-PET/CT was found to successfully detect and pinpoint metastatic lesions with high positive predictive value, regardless of their location in the body, in men with biochemically recurrent prostate cancer who had negative or equivocal baseline imaging. Higher positive predictive values were observed in extra-pelvic lymph nodes and bone compared to soft tissue regions.