A treatment planning class solution for hipppocampal avoidance whole brain irradiation using volumetric-modulated arc radiothera
Images

Whole brain radiotherapy (WBRT) remains the primary treatment option for patients with multiple brain metastases.1 Although the conventional method of using opposed lateral beams for WBRT can achieve uniform dose distribution over the whole brain, the high radiation dose to the hippocampus may result in neurocognitive function (NCF) decline.2,3 Using intensity modulated radiotherapy (IMRT), a phase IIRadiation Therapy Oncology Group (RTOG) trial (0933), was designed to study clinical feasibility in hippocampal sparing during WBRT.
Several groups have reported technical feasibilities in implementing HA-WBRT. Gutiérrez et al evaluated hippocampus avoidance (HA) WBRT (HA-WBRT) using tomotherapy with a simultaneous integrated boost to brain metastases, while achieving homogeneous dose distribution in the whole brain (equivalent to conventional WBRT) and sparing the hippocampal regions.4 Using a volumetric arc therapy (VMAT) technique, Hsu et al reported a planning study of HA-WBRT, while simultaneously boosting one to3 brain metastases to 63 and 70.8 Gy.5 The mean delivery time of these VMAT plans was 3.6 minutes. Using the same VMAT method, Awad et al reported their clinical experience on 30 patients with median whole brain dose of 31 Gy and a boost dose to the brain metastases of 51 Gy.6 They reported that the treatment was clinically feasible and tolerable. The mean time to delivery was about 3.43 minutes compared to 1.3 minutes for the conventional whole brain treatment. Nevelsky et al evaluated the feasibility of HA-WBRT using the Elekta Infinity linear accelerator and Monaco treatment planning system with a nine-field configuration and step-and-shoot delivery method.7 They achieved planning goals defined by the RTOG 0933 protocol. In this study, we reported our planning and delivery experience of VMAT for HA-WBRT under a mixed vendor environment, using the Pinnacle treatment planning system v9.0 by Philips Healthcare, while delivering treatment on Elekta Synergy and Novalis-TX linear accelerators.
Planning
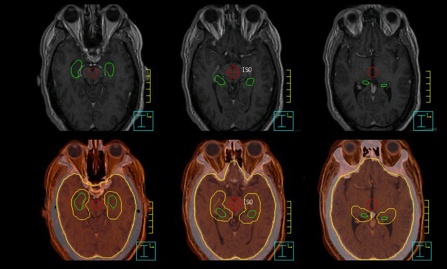
Ten patients, who received WBRT for brain metastases at our institution, were randomly selected in this study. The computed tomography(CT) images with 2-mm slice thickness were acquired on a Philips Brilliance Big Bore 16-slice CT simulator, and then exported to the Pinnacle treatment planning system. To facilitate contouring, magnetic resonance imaging (MRI) scans (axial T2-weighted and T1-weighted contrast enhanced MP-RAGE) with 1.5-mm slice thickness were imported and registered with the planning CT images in Pinnacle. Following the guidelines in the RTOG 0933 protocol, a radiation oncologist contoured clinical target volume (CTV), hippocampus, and other organs-at-risk (OAR), such as the lenses, eyes, optic nerves, and brainstem based on MR/CT images (Figure 1). The planning target volume (OTV) was constructed as the CTV (whole brain), excluding the hippocampal avoidance, which was generated with a 5-mm uniform expansion from the contoured hippocampus.
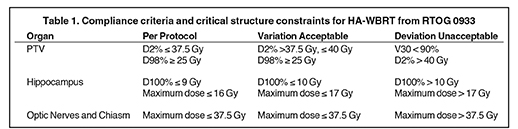
For each patient, 2 VMAT plans were created in Pinnacle for a Novalis-TX and an Elekta Synergy-S system. The Novalis-TX linear accelerator is equipped with 60 leaf pairs of high-definition (HD) multileaf collimator (MLC). With HD-MLC, 32 inner leaf pairs and 28 outer leaf pairs have a respective projection leaf width of 2.5 mm and 5.0 mm at the isocenter plane. The Synergy-S system is equipped with a MLC consisting of 40 leaf pairs of a 4.0-mm projection leaf width at the isocenter plane. All VMAT plans were generated using SmartArc optimization with 2 arcs: a 358° arc (from 181º to 179º) and another 200° (from 100º to 260º) arc. To minimize the dosimetric effect of the tongue-and-groove in the MLC, the collimator angle of 2 arcs was set to 30° or 45°. Other important optimization parameters were: the maximum iterations of 30,the convolution-dose iterations at 15, the final gantry spacing of 4º, and the maximum delivery time of 90 seconds. The maximum dose rate on each system was set to 400 MU/min. The planning acceptance criteria were to achieve the planning goals recommended by the RTOG 0933protocol (Table 1).
Because the optimization used in the Pinnacle is a gradient search method, it is likely the optimized solution could be trapped in a local minimum, especially for VMAT plans involved with a concave-shaped target, such as the PTV in the HA-WBRT case. Therefore, the finalplan quality from the Pinnacle has a large variation, heavily depending on how the planning objectives were added to guide the computer optimization. A typical set of planning objectives cannot be directly applied and multiple manual tunings of the planning objectives are required.To expedite the planning process, we developed a progressive approach of how to manually adjust the planning objectives for each OARs and the PTV. An example of the planning objectives for this progressive method is listed in Table 2. As shown in Table 2, we started the planning objectives with only PTV coverage in the first optimization. After this optimization, the VMAT plan achieved a uniform dose distribution with excellent dose coverage to the entire brain (or the PTV), but no sparing of the hippocampus avoidance or other OARs. Without resetting the beams after the first optimization, we manually increased the weighting factors to the hippocampus avoidance and other OARs, as shown in Table 2. Because of the nature of the gradient search method, after the second optimization, the dose coverage to the majority of the PTV was maintained while the doses to the hippocampus and other OARs were successfully decreased. However, the maximum dose in the PTV was increased and the percent volume of the PTV receiving the prescription dose was also decreased. To recover PTV dose coverage, the weighting factors in both PTV and hippocampus avoidance were manually adjusted as shown in optimization 3 in Table 2. Such adjustments may continue several times until the VMAT plan meets the plan acceptance criteria in Table 1.
Results
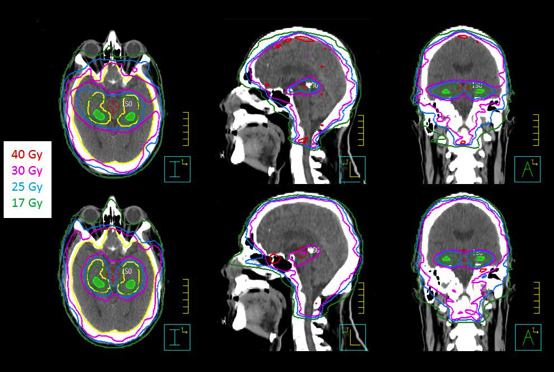
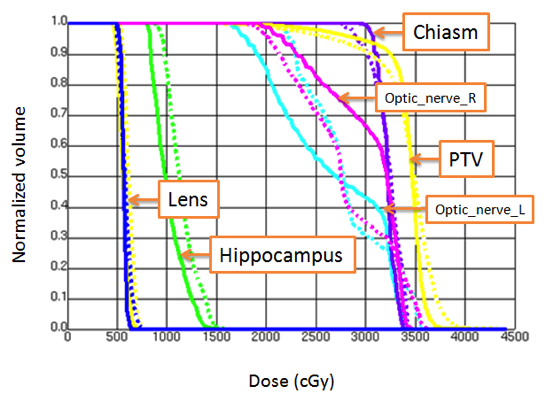
All VMAT plans achieved the plan acceptance criteria (dose compliance) per RTOG 0933 protocol or with acceptable variances. Figures 1and 2 show an example of dose distributions and dose volume histograms from 2 VMAT plans (Novalis-TX and Synergy-S) for the same patient.With ≥ 90% of the PTV receiving 30 Gy, the average volume of the PTV receiving 25 Gy (37.5 Gy) was 96.9 ± 1.0% (0.7 ± 0.9%) and 96.2 ±0.7% (1.8 ± 0.9%) for the Novalis-TX and Synergy-S VMAT plans, respectively. For the hippocampus, D100% and the maximum dose were 7.9 ± 0.5 Gy and 15.3 ± 1.3 Gy for the Novalis-TX plans; D100% and the maximum dose were 8.3 ± 0.3 Gy and 15.4 ± 1.0 Gy for the Synergy-Splans. The total MUs for Novalis-TX and Synergy-S machine was 889 ± 109 and 1,157 ± 127, respectively. We noticed that the VMAT plans for Novalis-TX had improved the plan quality compared to the VMAT plans with the Synergy-S plan, partly due to the smaller leaf width for the Novalis-TX linear accelerator.
Conclusion
We developed a planning class solution for hippocampus avoidance of whole brain radiation using planning and delivery systems with mixed vendors. Following the RTOG 0933 protocol and our planning class solution, the treatment plan for the hippocampus avoidance of the whole brain radiation can be completed within 2 to 4 hours after completion of all contours.
References
- Sundstrom JT, Minn H, Lertola KK, Nordman E. Prognosis of patients treated for intracranial metastases with whole-brain irradiation. Annals of medicine. 1998;30(3):296-299.
- Aoyama H, Tago M, Kato N, et al. Neurocognitive function of patients with brain metastasis who received either whole brain radiotherapy plus stereotactic radiosurgery or radiosurgery alone. International journal of radiation oncology, biology, physics. 2007;68(5):1388-1395.
- Li J, Bentzen SM, Li J, Renschler M, Mehta MP. Relationship between neurocognitive function and quality of life after whole-brain radiotherapy in patients with brain metastasis. International journal of radiation oncology, biology, physics. 2008;71(1):64-70.
- Gutierrez AN, Westerly DC, Tome WA, et al. Whole brain radiotherapy with hippocampal avoidance and simultaneously integrated brain metastases boost: a planning study. International journal of radiation oncology, biology, physics. 2007;69(2):589-597.
- Hsu F, Carolan H, Nichol A, et al. Whole brain radiotherapy with hippocampal avoidance and simultaneous integrated boost for 1-3 brain metastases: a feasibility study using volumetric modulated arc therapy. International journal of radiation oncology, biology, physics. 2010;76(5):1480-1485.
- Awad R, Fogarty G, Hong A, et al. Hippocampal avoidance with volumetric modulated arc therapy in melanoma brain metastases - the first Australian experience. Radiation oncology. 2013;8:62.
- Nevelsky A, Ieumwananonthachai N, Kaidar-Person O, et al. Hippocampal-sparing whole-brain radiotherapy using Elekta equipment. Journal of applied clinical medical physics / American College of Medical Physics. 2013;14(3):4205.