Expediting the treatment planning process
Images
Radiation treatments today can be delivered in a matter of minutes, yet treatment planning continues to be the cog in the wheel slowing down therapy. Within the last year, however, new developments in treatment planning solutions, such as autocontouring and predictive modeling, are streamlining the more time-consuming steps to expedite the overall process.
Key challenges
There are two key challenges in treatment planning—speed and accuracy.
It can take several hours to precisely contour and calculate a dose plan for complicated cases.
“Speed of treatment planning is currently a problem and even the best dosimetrists have a problem with consistency. Historically, treatment planning has been really more of an art than a science; dosimetrists need years of planning experience to gain an intuition of what is possible to provide to individual patients in terms of delivered doses,” said Sasha Mutic, PhD, director, Clinical Medical Physics, professor, Radiation Oncology, Mallinckrodt Institute of Radiology at Washington University School of Medicine, St. Louis, MO.
Although dosimetrists strive to optimize the dose plan while minimizing damage to surrounding structures, unknowingly, they may fall short of reaching this goal.
“Commonly, planners try different parameter values to drive the optimizer in the direction they want, and stop when they feel they have done as well as they could. Some plans approach the ‘optimal frontier,’ but many approved plans are far from optimal,”said Kevin L. Moore, PhD, DABR, Assistant Professor, Department of Radiation Oncology, University of California, San Diego,CA. “When you don’t know what the absolute best plan is for the patient, you can waste a lot of time, or stop before you’ve spared the organs at risk as much as possible.”
Because contouring variability is a major source of uncertainty in radiotherapy treatment planning, it has become a focus of research, with emphasis on both planning target volumes (PTV) and organs at risk (OAR) for many anatomical sites.1-5 Recently, a number of innovations in treatment planning technology have provided new approaches to overcoming obstacles related to speed and accuracy, with the potential to advance the science by leaps and bounds.
Making trade-offs
In radiation therapy, clinicians have to make trade offs between target coverage and organ sparing, and between speed and accuracy.
Speed is often sacrificed for accuracy. Depending on the complexity of the anatomical site being treated, planning for intensity-modulated radiation therapy (IMRT) can range from a couple of hours to a couple of days, says Michele Verst, MS, chief medical physicist, Union Hospital’s HUX Cancer Center, Terre Haute, IN.“There are two challenges in the treatment planning process that go hand in hand—one is developing the most accurate plan from a dosimetric standpoint as far as dose calculation and how it affects what’s truly being delivered to the patient,” said Dr. Verst.
A significant advancement in accuracy came with the implementation of Monte Carlo calculations into a planning system. “The MonteCarlo algorithm at this point is the most accurate way to predict how the dose is being delivered inside the patient,” indicated Dr. Verst. “Asa trade off, to get that accuracy, it requires a lot of time. It is very time consuming to make those calculations with that type of precision.”
The HUX Cancer Center is a pilot site for the recently FDA 510(k)-cleared version Monaco 5 treatment planning system by ELEKTA. Asa pilot site, Dr. Verst compared the legacy system to the new version of Monaco. “We can do the same or better quality plan with Monaco injust 2 to 3 iterations compared to 8 or 10 iterations,” she said. “What Monaco has been able to do is merge the best of both worlds. It solves two different challenges: one is getting an accurate view of what’s going on inside the patient, and two, giving you a good plan within a reasonable amount of time.”
Monaco 5 supports a full spectrum of radiotherapy techniques, including volumetric-modulated arc therapy (VMAT), IMRT, and 3-dimensional (3D) conformal radiation therapy. It also is equipped for stereotactic radiosurgery (SRS) and stereotactic radiotherapy (SRT).“The software has gone from using standard constraints for your prescription or your dose limitations for your OARs toward more of a biological model with Monaco. For example, the serial and parallel functions are combined along with some other maximum constraints to give you the flexibility to use how the structures function physiologically,” indicated Dr. Verst. “You can set it so the maximum part of the structure can get no more than 2400 cGy. But we can say a third of the liver can get a certain amount of dose, while the remaining two-thirds gets another dose. It helps you to tailor the plan specific to the site, allowing you to do dose painting on your target volume.”
Additionally, the Segment Shape Optimization feature on Monaco generates an ‘ideal plan’ with the dose and constraints the user selects.“It allows you to use the strengths of a particular linac to give you the best possible option for delivering the ideal plan that you would like to give. That’s one really nice tool,” said Dr. Verst.
Optimized contouring
Contouring is a labor-intensive and time-consuming step in the treatment planning process and tends to be highly variable. Some of the quality control tools include RTOG contouring protocols, which are based on a consensus reached among cooperative groups and disease site committees, and are designed to provide treatment guidelines that include quality criteria for a specific type of treatment.
Another quality control measure is computer-assisted auto-contouring algorithms, such as automated atlas-based segmentation. These assist the dosimetrist in overcoming the limitations of manual contouring.6 Auto-contouring is a well-established technology in treatment planning systems. The Pinnacle3 treatment planning system by Philips Healthcare saves planning time and improves consistency by automating the contouring process. In its latest iteration, Pinnacle3 9.6, the Dynamic Planning feature provides a fast assessment to determine the need tore-plan and dynamically tracks the impact of patient changes to treatment plans. The fullAccess feature accelerates the plan review by providing the ability to review and annotate images or plans remotely.
Predictive models
Taking a step beyond autocontouring is knowledge-based treated planning. These algorithms contour anatomical images by using a mathematical model that predicts dose-volume histograms (DVHs) from a patient’s anatomy. This predictive model helps determine what theDVHs for each organ of interest should look like.
“Our approach is designed to make use of a database of treatment plans for previously treated patients. Using mathematical techniques, you can analyze IMRT dose distributions designed for patients, and correlate the different dose distributions to the differences in patient anatomy. Ultimately, this work helps you develop a mathematical model that predicts DVHs from a patient’s anatomy,” explained Dr. Moore, who worked on developing this model while at Mallinckrodt Institute of Radiology.
“Up to this point, there has never been a quantitative way to predict what the DVH should look like in a particular patient treatment based on past data from optimized treatment plans,” said Dr. Moore. “It determines, for example, if the current patient has a large target or smallOAR, and what the new plan DVHs should look like. As targets or organs grow or shrink, the model can predict how the DVHs change in response. If the OAR moves away from the target, the model will predict that the amount of dose reaching the organ will diminish.”
According to Dr. Moore, what contributes to variability in IMRT treatment plan quality is human error, such as estimating the DVH or omission of important data. The predictive model automates the process to make it more reproducible.
The clinical implications could dramatically reduce side-effects, indicates Dr. Mutic, who worked with Dr. Moore on the development of the knowledge-based treated planning solution.
“Predictive models will eliminate variability and help standardize the outcomes and complications across facilities,” said Dr. Mutic.“Currently, patients are not being treated the same or delivered the same amount of dose. Ensuring patients receive consistent dose will drive the quality of treatments that will lead to more consistent outcomes.”
A case in point is the treatment of head-and-neck cancer, for which there could be dramatic improvements in parotid gland sparing.“There is a very wide gulf between the doses that are called for in treating head-and-neck tumors, versus the doses that the parotid gland can tolerate. Researchers have observed a huge amount of variability from patient to patient in terms of how well dose to the parotid glands was effectively minimized,7” said Dr. Moore.
“When we compared plans created with and without the use of a predictive tool, the differences between them were incredibly dramatic.We saw much less variability plan to plan after we had the predictive model, and the average deviations from the predictions were much smaller. The number of patients whose planned doses exceeded tolerance levels was categorically reduced,” he said.
The key connecting step with the predictive model is that the data with the predictions is automatically input directly into an IMRT optimizer that is designed to make use of them.
“Instead of having humans punch in numbers based on the average patient or a clinician’s intuition, they can work with precise expected values and use them to guide the optimization,” said Dr. Moore.
For the patient, reduced variability among treatment plans means less damage from dose distributed to surrounding healthy structures.
Similarly, Varian Medical Systems (Varian) offers a knowledge-based solution that uses predictive modeling for treatment plans. Varian has recently received FDA 510(k) clearance for its RapidPlan software, which is designed to provide standard-of-care models to use as a baseline for developing new IMRT treatment plans. Clinicians can select their best treatment plans to include in a training set that can be used to create new and improved practice models in the future. In doing so, sites can customize RapidPlan to reflect their own practices. The models can also be shared among colleagues within a care network to create a practice standard.
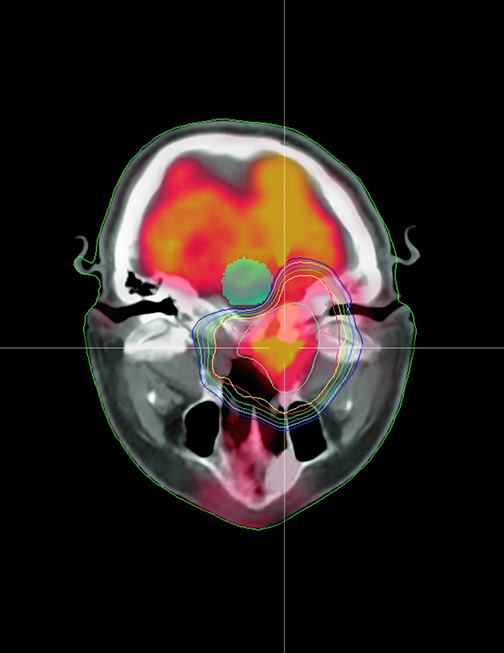
RapidPlan is a comprehensive tool within Varian’s Eclipse treatment planning system that may be used to plan external beam radiotherapy,including intensity-modulated radiotherapy (IMRT), image-guided radiotherapy (IGRT), RapidArc radiotherapy, stereotactic body radiotherapy (SBRT), and stereotactic ablative radiotherapy (SABR) (Figure 1). The solution is integrated with Varian’s Eclipse treatment planning software.
Flatten the learning curve
Key to knowledge-based technology is that it is a learning system that will allow inter-institutional collaboration and benchmarking.
“Clinics will be able to ‘train’ their own models. The technology gives local institutions the power to develop their own stereotactic liver radiotherapy model, for example. The automated planning component of it will be based on exactly what clinicians want to do at a local institution,” explained Dr. Moore.
RapidPlan is also a learning system. Clinicians can take their best treatment plans and add them to the system for use in creating new, improved practice models for the future. The models can be shared among colleagues within a care network to create a practice standard.
Collaboration among institutions will be greatly facilitated with a knowledge-based solution so widely available. Users will be able to share data without needing approval from the Institutional Review Board or expending valuable time planning benchmark cases.
“Institutions can base models on the RTOG protocols, or share them with others,” Dr. Moore said. “You have a means to compare your output to other institutions or gold standard datasets, for example, coming out of a national clinical trial or a large academic institution that provides its own models. Everyone can make use of that—you flatten out the learning curve.”
The sharing of data helps clinicians become familiar with newer techniques. Dr. Moore points out that many radiation oncologists are reluctant to move into linear accelerator-based SRS, for example, because the constraints and fractionations are unfamiliar. “This could be a technology that allows them to immediately benchmark their first 10 plans against exemplary work done at established academic institutions,” he noted. “There is tremendous potential in terms of what this technology could do for the field in terms of sharing data.”
Leveling the playing field
When making the choice, most clinicians would put accuracy before speed. Historically, TomoTherapy’s TPS has been considered one of the most conformal treatment planning systems. A recent study found the overall treatment plan quality using TomoTherapy was better than the other TPS technology combinations.8 Yet it was not faster when compared to Varian’s Eclipse, ELEKTA’s Monaco or Pinnacle3 byPhilips Healthcare.
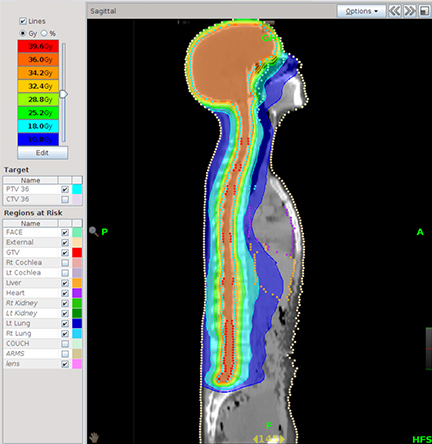
Recently, however, the TomoTherapy system got an overhaul that has given it an edge not just in accuracy but also in speed. In October of2012, Accuray Inc. launched its new TomoTherapy H Series, including the TomoHDA System, designed with faster planning, faster delivery,and increased quality. Some of the key features of the TomoHDA system include TomoEDGE Dynamic Jaws technology, designed to provide added flexibility in treatment delivery by sharpening dose fall off and accuracy. TomoEDGE Dynamic Jaws technology combined with VoLOPlanning, a graphics processing unit (GPU)-based treatment planning solution, enables high-speed parallel processing for both dose calculation and optimization (Figure 2). VoLO leverages advanced graphics processing technology and a new calculation algorithm to significantly reduce treatment-planning times and add flexibility in developing even the most complex radiation therapy plans.
The Tulsa Cancer Institute, a large site that treats anal, rectal and gynecological diseases, as well as lung, brain, head and neck and the spine, has 2 TomoTherapy units on site. According to Matthew West, PhD, chief physicist at the Tulsa Cancer Institute, efficiencies ar egained in the overall workflow.
“When you look at the efficiencies, the system is very simple. Unlike a conventional accelerator, there are no ancillary components or special modes for special types of treatments, so whether you’re planning a prostate, a brain, breast or stereotactic case, you plan it and treat them the same. So the efficiencies come in terms of ease of workflow and safety,” said Dr. West. “The treatment data isn’t transferred from one computer to another, but is verified by physics prior to treatment. Ultimately, this attention to patient setup and image guidance allows clinicians to reduce treatment margins and minimize dose to critical structures.”
The introduction of the VoLO treatment planning system has significantly accelerated the overall process. “The VoLO part has sped up the time it takes to turn around a treatment plan. Previously, on the older system without VoLO, it could take between 30 minutes and up to 4 hours depending on how complicated it is. Now, on the system with VoLO, it only takes 2 minutes before we start planning,” indicated Dr. West.
Similarly at Cancer Healthcare Associates in Miami, FL, Martin Keisch, MD, president and medical director, appreciates how VoLO cuts time-consuming steps in the process, such as record and verify.
“What appealed to me about TomoTherapy is the treatment planning system and the treatment delivery software is on a unified platform. So you don’t have the record and verify software between the treatment planning system and treatment delivery, and the process is shortened by an hour or 2,” said Dr. Keisch. “Once I complete the plan, the second I close out, the plan is already on the treatment workstation and ready to pull up.”
A critical step in treatment planning is meeting predefined goals and establishing an end point. As Keisch explains, the speed with whichVoLO meets initial set goals can be as fast as 5 to 10 minutes. This allows for additional time to continue setting more stringent criteria, such as lower dose to critical structures, or evening out doses distributed to the tumor or the target.
Another step that VoLO eliminates is predetermining an angle for the TomoTherapy system because it looks at all angles continuously.“That intermediate step no longer exists, and that’s what really makes it fly,” said Keisch.
The VoLO TPS provides a protocol library that allows users to customize and adjust anatomical structures, in addition to a library of constraints for the normal tissues and library of goals for the target volumes.
“When you load the plans it prompts you to pull in all of those criteria, plus the jaw size, the pitch, and the fineness of dose calculation matrix,” indicated Keisch. “Now for a prostate, I can literally get a good plan in 5 minutes of calculation time. With the head and neck, I can get a good calculation in 10 minutes.”
Patients treated on TomoTherapy often receive a boost on Accuray’s CyberKnife Robotic Radiosurgery System, a noninvasive alternative to surgery for the treatment of both cancerous and noncancerous tumors. CyberKnife’s Multiplan treatment planning solution will soon support integrating treatment plans across the 2 platforms.
“You will be able to import plans from TomoTherapy into Cyberknife and adjust the plans to do a boost on Cyberknife. It is common to boost a tumor to a higher dose to treat lymph nodes,” explained Scott MacDonald, medical dosimetrist, Accuray Inc.
“You can also create a contouring library in the Templates feature on the existing Multiplan system. There is also the Sequential Optimization algorithm, which is based on RTOG recommendations and automatically calculates how to avoid critical structures,” addedMacDonald.
While treatment plan integration has not yet been released, CyberKnife’s Multiplan currently includes AutoSegmentation, which automatically generates contours for intracranial and male pelvic anatomy using both model-based and atlas-based delineation methods.
“Autosegmenting for most commonly used applications helps speed up throughput,” said MacDonald. “It gives you flexibility on complex plans by autocontouring multiple objects—10 contours within a few minutes.”
On the current Multiplan platform, the QuickPlan feature automates the entire planning process, including setting planning parameters and dose calculations. While Sequential Optimization develops tailored treatment plans specific to clinical objectives for each patient, the system also uses Monte Carlo Dose Calculation, often considered the gold standard for dose calculation, to rapidly develop plans. Finally, the 4D treatment optimization tool takes into account the movement of the target and the movement and deformation of the surrounding healthy tissue and critical structures.
Art to science
In striking a balance between optimizing dose and limiting side effects, dosimetrists have historically relied in part on intuition. Yet, as new technologies continue to streamline treatment planning, the process is becoming less of an art and more of a science.
References
- Barghi A, Johnson C, Warner A, et al. Impact of contouring variability on dose-volume metrics used in treatment plan optimization of prostate IMRT. Cureus. http://www. cureus.com/articles/2348#.Uop_amRgZRg.
- Vorweck H, Beckmann G, Bremer M, et al.: The delineation of target volumes for radiotherapy of lung cancer patients. Radiother Oncol. 2009; 91:455-460.
- Petersen RP, Truong PT, Kader HA, et al.: Target volume delineation for partial breast radiotherapy planning: clinical characteristics associated with low interobserver concordance.Int J Radiat Oncol Biol Phys. 2007;69:41-48.
- Yamamoto M, Nagata Y, Okajime K, et al.: Differences in target outline delineation from CT scans of brain tumours using different methods anYamamoto M, Nagata Y, Okajime K, et al. Differences in target outline delineation from CT scans of brain tumours using different methods and different observers. Radiother Oncol. 1999;50:151-160.
- Rasch C, Barillot I, Remeijer P, Touw A, van Herk M, Lebesque JV: Definition of the prostate in CT and MRI: a multi-observer study. Int J Radiat Oncol Biol Phys. 1999; 43:57-66.
- Lui E, Barghi A, Rodrigues G. Assessment of Multiple Atlas-Based Segmentation in Prostate Bed Contouring. Cureus. http://www.cureus.com/articles/2188#.UovMJ2RgZRg. Published April 3, 2013. Accessed November 18, 2013.
- Moore KL, Brame RS, Low DA, Mutic S, et al. Experience-based quality control of clinical intensity-modulated radiotherapy planning. Int J Radiat Oncol Biol Phys. 2011;81:545-551.
- Wiezorek T, Brachwitz T, Georg D. Rotational IMRT techniques compared to fixed gantry IMRT and Tomotherapy: Multi-institutional planning study for head-and-neck cases. Radiat Oncol. 2011 Feb 21;6:20. doi: 10.1186/1748-717X-6-20.