Treatment planning systems: Balancing standardization with personalization
Images
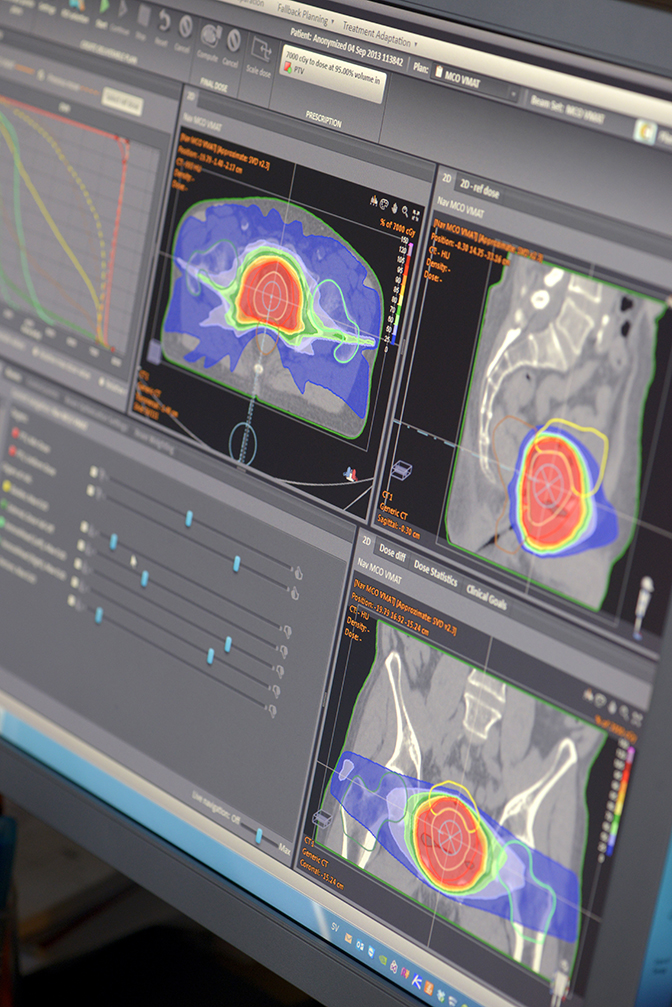
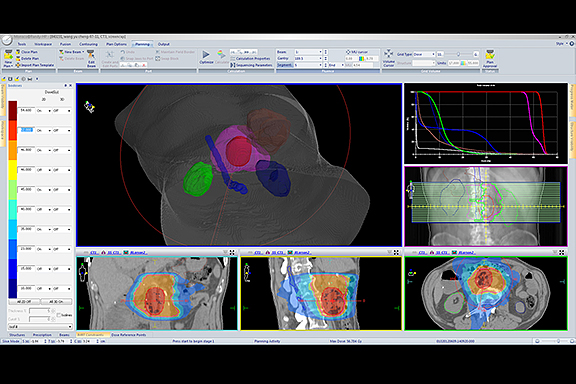
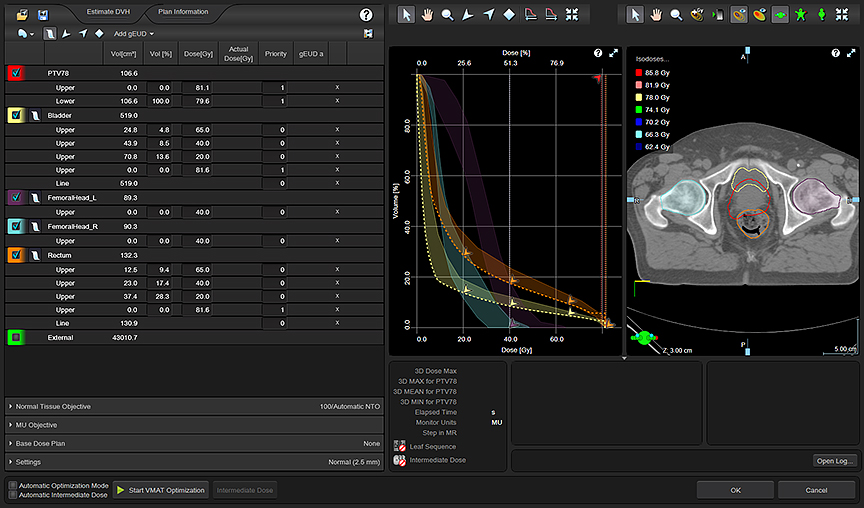
Advanced technology continues to reshape the field of radiation therapy (RT), most notably with improvements in the precision of therapy delivery. Image-guidance during treatment is now possible, including the real-time tracking of moving tumors such as those in the lungs or abdomen. Beam modulation has helped open the door to new techniques like volumetric modulated arc therapy (VMAT), which optimizes the plan in many angles, and then sequences it into stacks of apertures at every angle followed by delivery of the beam with multiple connected arcs. Stereotactic body radiation therapy (SBRT) is another emerging treatment plan that localizes the lesion and delivers limited, yet precise, high-dose radiation—often in a single high dose or in a few fractionated treatments.
An integral component for achieving these advanced RT delivery schemes remains the treatment planning system (TPS), the “brains” of modern day RT. The continued evolution of computerized solutions and image-guidance has helped reduce the morbidity and toxicity of cancer treatments. Enhancements to automated planning, consistency in planning across patients and institutions, robust algorithms, and quantitative knowledge-based planning will further advance physicists’ ability to generate high-quality, efficient treatment plans.
Balancing best practices with personalized medicine
Reducing variability to provide consistency in care is an important consideration in any TPS. “We follow protocols where we can, but in some cases there is an issue that prevents us from achieving a certain goal,” says Jeremy Donaghue, MS, DABR, chief medical physicist at Akron General Medical Center in Ohio. “That’s where personalization comes in.”
Using a multi-criteria optimization (MCO) technique available in the RayStation TPS (RaySearch Labs, Stockholm, Sweden, and Garden City, New York), Donaghue can evaluate different scenarios based on various requirements. He starts with certain anchor points that define the plan. Then, by adjusting additional elements, he can see what the impact will be. For example, in a prostate plan Donoghue can balance out the type of coverage delivered near critical structures such as the rectum and the bladder. Using MCOs helps him reduce variability, which leads to greater consistency in care across patients with similar disease.
“MCO helps me determine the best plan that I can get,” explains Donaghue. “Even if I can’t achieve all that I want in a plan, it helps me know the limitations. Using this tool streamlines the plan for the physicians and the dosimetrist. Even though we want to try and treat each patient similarly, it helps me personalize it to their specific anatomy so I can turn around the plan more efficiently.”
Donaghue also uses a scripts feature to compare and standardize data across different patients. This allows him to take data from similar patients and create an average and standard deviation. He can then compare a plan to the standard deviation and identify segments that fall outside that norm.
At Kettering Medical Center, Kettering, Ohio, Christopher M. Wennerstrom, MS, DABR, medical physicist, agrees that achieving the right balance between standardization and personalization is important. At his facility, he uses Monaco (Elekta, Atlanta, Georgia) to help build a solid starting point in treatment planning.
“Efficiency and a good class solution lead to better care, not only for that particular patient, but it also further affects the plans for others,” he says. By efficiently enhancing that specific starting point for a certain type of treatment plan, he can spend additional time on more complicated plans.
“Class solutions help me to develop best practices, so there isn’t mass variability. Yet it provides the flexibility for individualized medicine,” he adds. For example with Monaco, when he brings in a template for a VMAT SBRT lung plan, the template is expecting a set of contour names. However, if he has changed one descriptor, for instance a specific planning target volume (PTV), he can select that one contour name in the prescription from a drop-down menu without invalidating the entire class solution.
The calculation algorithm’s robustness is most important, adds Wennerstrom. “As planning systems have evolved and use more robust algorithms, the difference between what we are seeing on the screen and reality is becoming smaller. However, the tradeoff for that is often calculation speed. Moving forward, what we need from our vendors is the most robust, best planning algorithms with as much speed and computational power as possible. What we definitely don’t need is speed without accuracy.”
For the last several years, Kevin Moore, PhD, DABR, assistant professor, Department of Radiation Medicine and Applied Sciences at the University of California, San Diego, has researched ways to predict and quantify when a treatment plan can be improved. “This is a hugely underappreciated problem,” he says. While patient privacy regulations and competition between cancer centers impact TPS data sharing, patients should not be getting vastly different treatment plans from different treatment centers, Dr. Moore adds.
Using quantitative, patient-dependent benchmarks is the core of best practices, but is not inherent in today’s TPS, he states. “They are still designed to prepare a plan for a single patient,” says Dr. Moore, “not to help you learn a larger sense about cohorts of patients.”
Dr. Moore has licensed some of his research and work to Varian Medical Systems (Palo Alto, California) on synthesizing prior patient treatment plans into predictive models that help automate and optimize future treatment plans for use in RapidPlan, a knowledge-based planning system that allows clinicians to develop and apply best practice models for automated planning. While he says RapidPlan is a step in right direction and provides a quality control baseline, there is room for improvement across all vendors’ TPS products, “as evidenced by published studies that show wide variability and suboptimal planning,” Dr. Moore says.
Trends and unmet clinical needs
Variability across patients should be eliminated in the treatment planning process, says Dr. Moore. The motivation for much of his work in developing knowledge-based treatment planning based on statistical learning of past experiences is to help further reduce complications in current and future plans. These quantitative predictions will help physicists develop quality, standardized plans that also allow for personalization.
He would like to see this type of work extended across institutions. That knowledge will help account for clinical tradeoffs in a way that doesn’t digress too far from an optimal plan. The goal is to ensure the plan adapts to tradeoffs in a manner that is consistent across plans.
“The ability to perform aggregate studies and give the user the ability to perform queries across multiple patient treatment plans with novel questions is not something today’s TPS [is] designed to do,” Dr. Moore adds. “With statistical learning, week by week and year by year, we can expand that knowledge base to understand the average of what we are doing and planning on a larger scale.” Today, this is a manual process, but Dr. Moore believes that including these types of tools in a TPS that enables cross-institutional collaborative planning could be an element of the modern radiation oncology department.
Donaghue sees a movement by the industry to provide a one-stop shop for all treatment planning needs. For example, he can now perform deformable registration within his TPS solution, and he anticipates that his vendor will provide capabilities for brachytherapy in the near future. More adaptive planning and integrated record-and-verify tools are also on the rise, says Dr. Moore.
One limitation Donaghue would like addressed is for a TPS to provide a check for minor/obscure parts of AAPM TG 53 (American Association of Physicists in Medicine Task Group 53). TG 53 provides a framework for physicists to develop and implement a comprehensive quality assurance program that encompasses image-based definitions of patient anatomy, 3D beam descriptions for complex beams including 3D MLC apertures, 3D dose calculation algorithms, and complex plan evaluation tools, including dose-volume histograms.1
Wennerstrom also says the ability to develop multiple types of plans in one TPS—from 3D conformal to VMAT—will continue. Specifically, the ability to generate SBRT plans that take into consideration the dose levels of prior treatments is important, he says. Many departments that start offering SBRT will find that the number of patients being re-treated with this type of therapy will be higher than expected, he notes.
“With SBRT, what we used to know by heart about dose to critical structures goes out the window,” Wennerstrom says. “It is difficult to deal with dose subtraction, how much dose we have to play with in a structure using the current dose scheme, or the dose fractionation that we are trying to deliver, and justify how much dose is left for that critical structure.”
As TPS technology evolves, the industry will develop novel ways to deal with these issues, whether it’s via the biologic effective dose or another method. “We’ll need to get to a point where we can add dose together from different dose fractionation schemes and have it be an accurate reflection of the remaining dose that a critical structure can safely absorb,” says Wennerstrom. “A centigray is not the same when it is delivered in a higher fractionation.”
With a high efficacy and good outcomes, it’s no wonder that SBRT is gaining momentum. “SBRT is making targets treatable that weren’t previously treatable with high doses that are very targeted,” he adds.
“We can’t simply multiply the dose across the entire treatment region by one number. Different critical structures and tumors respond differently to the same dose. Assuming that we could correctly recalculate those doses, while respecting the radiobiology and physiology of each structure, would be a better solution within the TPS to use all of that dose voxel information.”
It is seems clear that automated planning coupled with a knowledge-based approach are key components enabling more efficient plans that reduce variability between patients. While barriers remain, namely across different treatment plans and institutions, that divide appears to be slowly closing.
Reference
- Frass B, Doppke K, Hunt M, et al. American Association of Physicists in Medicine Radiation Therapy Committee Task Group 53: Quality assurance for clinical radiotherapy treatment planning. Med Phys. Oct 1998; 25(10):1773-1829.